Abstract
Background
Dermoscopy devices can overcome the refractive properties of stratum corneum by interface medium or cross polarization such that the lesion can be easily seen.
Aim
To examine the dermoscopic feature in alopecia areata and correlate the severity of disease with dermoscopic features.
Materials and methods
Retrospective analysis of 72 patients suffering from alopecia areata (AA), irrespective of age and sex, who visited the dermatology outpatient department of a tertiary care center in Eastern India was carried out. The most recently developed cases of AA were examined dermoscopically. Variables included yellow dots (YDs), black dots (BDs), broken hair (BH), short vellus hair (SVH), and exclamation mark hair (EMH) on the basis of available literature and expertise.
Results
Yellow dots was the most common finding seen in 57 cases (79.16%), black dots in 51 cases (70.8%). Short vellus hair was seen in 32 cases (44.44%), broken hair was seen in 31 cases (43.05%), and exclamation mark hair in 23 cases (31.9%). YDs per field of vision was considered as the most common finding with increased severity of AA.
Conclusion
YDS, in increased number per field of vision, is the most consistent finding seen in severe cases of AA, as they are in progressive AA and alopecia universalis. An increased number of SVH and terminal hairs were seen in patients who were being treated.
Keywords: dermoscopy, alopecia areata, yellow dots, exclamation mark hair
Introduction
Dermoscopy is a noninvasive method that allows in vivo evaluation of microstructures of the epidermis, the dermoepidermal junction, and the papillary dermis otherwise not visible to the naked eye. Dry dermoscopy, also called trichoscopy, is ideal because it has a blocking filter against light reflection from the skin surface. Sometimes differentiating scarring alopecia from non-scarring alopecia is difficult and dermoscopy can be helpful at this stage. Characteristic dermoscopic features of AA are yellow dots, black dots, broken hairs, tapering hair (exclamation marks), and short vellus hairs [1–4].
Materials and Methods
A retrospective analysis of 72 patients suffering from alopecia areata, irrespective of age and sex, who visited the dermatology outpatient department was carried out. Institutional Ethical Committee clearance was obtained. All patients visiting the dermatology outpatient department were screened for AA. All patients with alopecia areata, including those under treatment, were included in the study. The clinical diagnosis of AA was established after a detailed history and clinical examination. Relevant investigations like 10% KOH preparation and biopsy was carried out in select cases. Thyroid profile was performed in all cases. The various patterns of AA were noted as patchy, diffuse, ophiasis, totalis, and universalis. The severity of AA was graded on the basis of the Severity of Alopecia Tool (SALT) score. Dermoscopy was performed using Dermlite II hybrid m (3Gen, San Juan Capistrano, CA) dermatoscope at 10X magnification in polarized mode and photographs were captured with an iPhone 6 (Apple, Cupertino, CA). Dermoscopy image capturing was performed by a single dermoscopist to ensure consistent photographs during the procedure [5]. Dermoscopic variables included yellow dots (YDs), black dots, broken hair, exclamation mark hair, short vellus hair and any other new findings. White dots were differentiated from yellow dots by three independent dermoscopists. In accordance with available literature and data, yellow dots and black dots were considered the most common variables for severity. Additionally an increased number of yellow dots, broken dots, or broken hairs per field of vision was considered as an increase in severity of AA.
Results
All patients were of the South Asian ethnic group and of Indo-Aryan race from northern India with mainly Fitzpatrick skin type 4 and 5 (dark brown color).
Age and gender
There were 41 male (56.94%) and 31 female (43.05%) patients. The male to female ratio was 1.3:1, with a mean age of 24.43 years, median 24, and range from 5–50 years. The most common age group for AA in our study was seen in the 21–40 year age group.
History and associated disorders
Family history of AA was positive in 5 cases (6.94%). Ten patients were positive for thyroid autoantibodies (13.88%). Coexisting autoimmune disease such as vitiligo was seen in one patient (1.38%) and psoriasis in one patient (1.38%).
Type of AA
Patients with progressive AA numbered 56 (77.77%), whereas 16 patients (22.22%) had non-progressive AA. Only scalp involvement was seen in 40 cases (55.55%). In the scalp, common sites involved were occipital (13 patients) and parietal regions (11 patients). Other sites involved were temporal (4 patients); frontal (4 patients); vertex (3 cases); whole scalp (5 cases); only beard involvement in 12 cases (16.66%); only eyebrow involvement in 1 case (1.38%); only eyelash involvement in 1 case (1.38%); ophiasis in 5 cases (6.94%); sisiapho in 3 cases (4.1%); alopecia totalis in 9 cases; and alopecia universalis in 5 cases (6.94%). Patchy alopecia was the most common type (50/72, 69.4%); single patch AA was discovered in 21 cases (29.16%); and multiple patch AA in 29 cases (40.27%) (Table 1).
TABLE 1.
Patient demographics. [Copyright: ©2017 Jha et al.]
| Age group (years) | Clinical Type of Vitiligo | |||||||
|---|---|---|---|---|---|---|---|---|
| Male | Female | Progressive AA | Non progressive AA | On therapy | ||||
| 0–20 | 21–40 | 41–60 | >60 | |||||
| 22 | 41 | 9 | 0 | 41 | 31 | 56 | 9 | 7 |
Yellow dots were seen in 57 cases (79.16%), black dots in 51 cases (70.8%), short vellus hair was seen in 32 cases (44.44%), broken hair was seen in 31 cases (43.05%), and exclamation mark hair in 23 cases (31.9%). The maximum number of yellow dots per field of vision were seen in all cases of alopecia universalis. The occurrence of YDs and BDs were seen together in 39 cases of progressive AA, YDs and BH in 31 cases, YDs, BDs and BH together in 23 cases. YDs and SVH in 19 cases, YDs and EMH in 19 cases (Figures 1–7).
Figure 1.
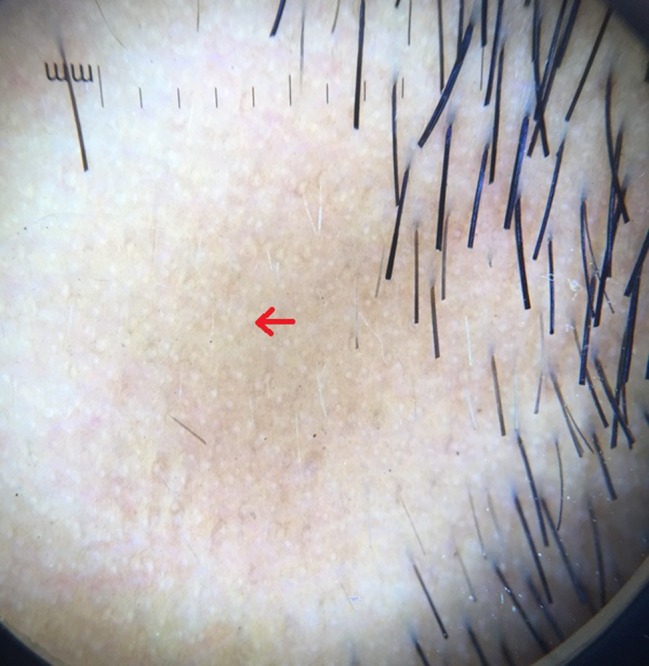
Red arrow showing yellow dots on dermoscopy. [Copyright: ©2017 Jha et al.]
Figure 2.
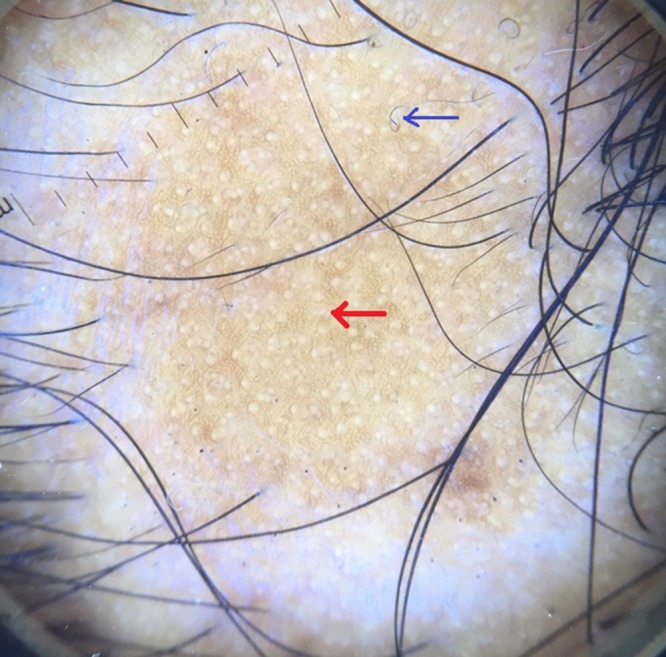
Red arrow showing yellow dots and blue arrow showing curly hair. [Copyright: ©2017 Jha et al.]
Figure 3.
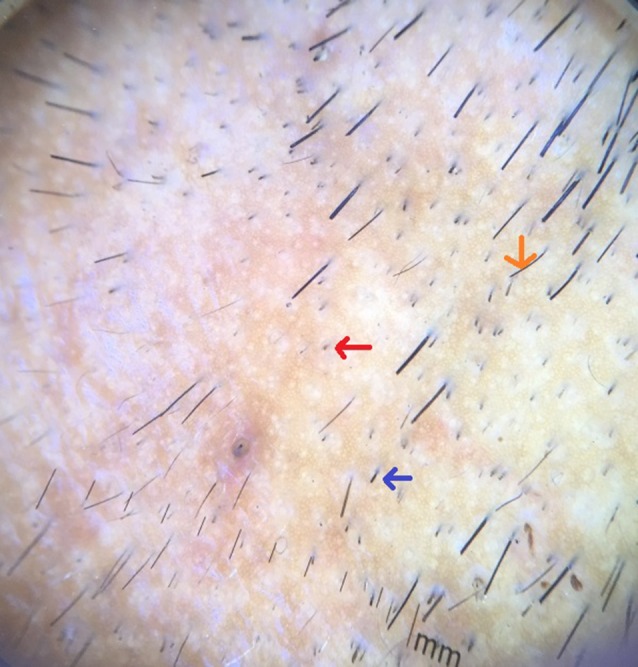
Red arrow showing black dots, blue arrow showing broken hair and orange arrow showing tapering hair. [Copyright: ©2017 Jha et al.]
Figure 4.
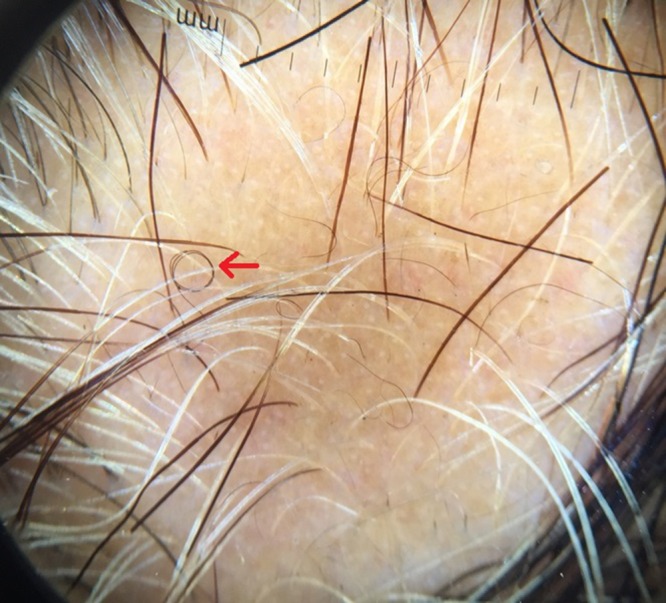
Red arrow shows curly hair. [Copyright: ©2017 Jha et al.]
Figure 5.
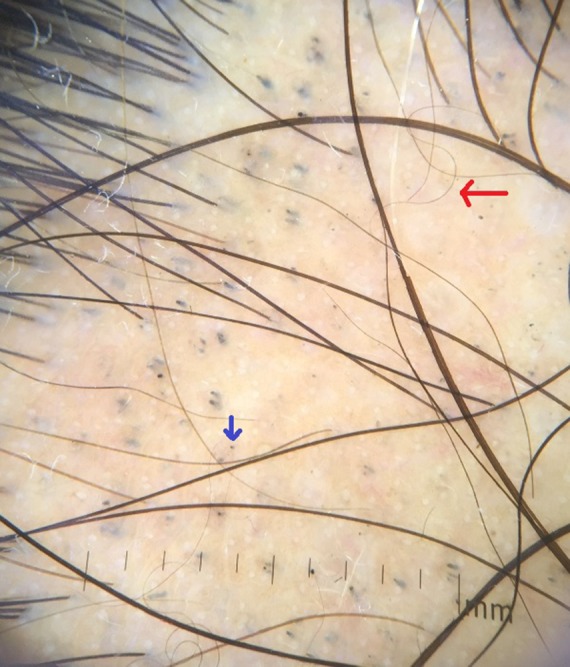
Blue dots showing black dot and red arrow showing curly hair. [Copyright: ©2017 Jha et al.]
Figure 6.
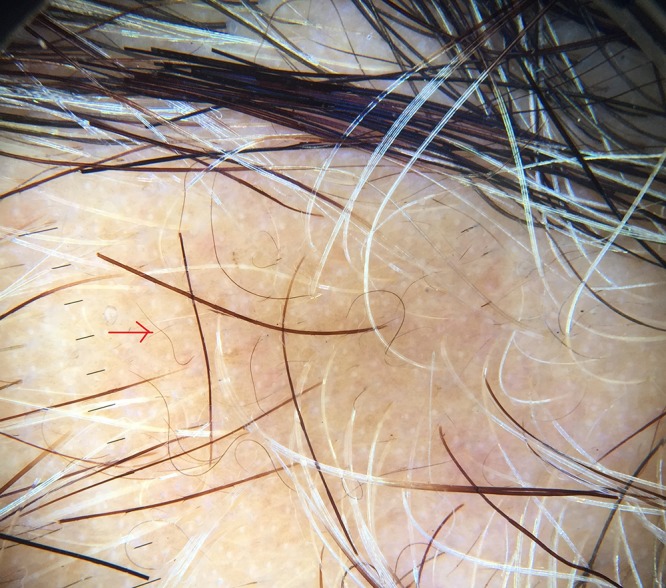
Red arrow showing short vellus hair. [Copyright: ©2017 Jha et al.]
Figure 7.

Yellow dots in alopecia universalis. [Copyright: ©2017 Jha et al.]
Treatment
Five patients were given intralesional triamcinolone acetonide. One patient with coexistent stable vitiligo was given bimatprost 0.03% ophthalmic solution, as there was presence of leukotrichia with the patch. An increased number of terminal hairs along with growth of SVH were seen in 5 out of 7 cases on treatment. White peripilar sign (Figure 8) was also seen in 4 out of 7 cases after treatment, with two to three hair units emerging from each follicle—a sign of hair regrowth.
Figure 8.
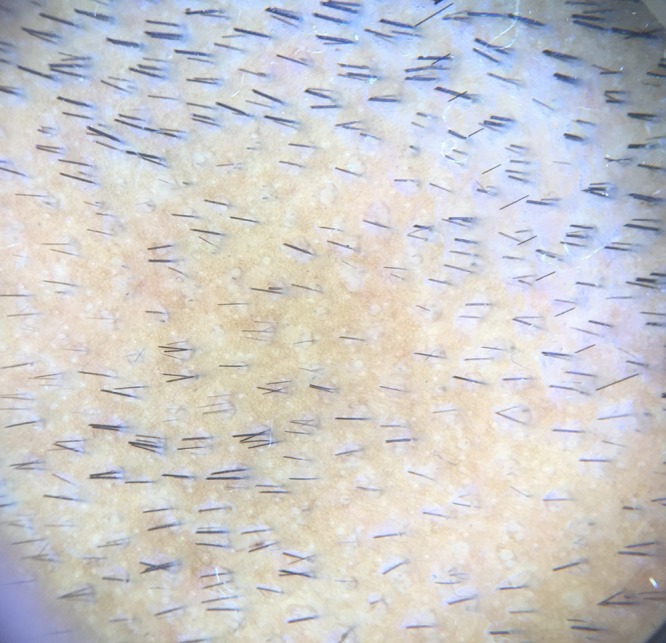
White peripilar sign seen in patients on treatment with two to three hair unit emerging from each follicle a sign of hair regrowth. [Copyright: ©2017 Jha et al.]
Discussion
A study done by Inui et al [2] of 300 patients with AA, in different stages and different subtypes of the disease, identified yellow dots, black dots, broken hairs, coudability hairs, and clustered short vellus hairs (<10 mm) as the most common features [6]. Yellow dots and short vellus hairs were the most sensitive markers for the diagnosis, and black dots, tapering hairs, and broken hairs were the most specific markers [2]. Yellow dots present as round or polycyclic yellow to yellow-pink dots that may be devoid of hairs or contain miniaturized, cadaverized, or dystrophic hairs. Although the yellow dots are sensitive (detected in all of 58 patients with different types of AA [6]), they correspond on pathology to the dilated infundibular ostia filled with sebum and degenerated follicular keratinocytes [1,7]. In our study, yellow dots (Figures 1, 2) were the most common finding seen in 57 cases (79.16%). In a study conducted by Inui et al [4], YDs were seen in 63.7% (191/300) of cases in contrast to Ross et al’s study, where 94.8% cases with AA had YDs (55/58 cases) [8]. Mane et al [3] reported an incidence of 81.8% among 66 patients and Hegde et al [9] reported the incidence of YDs was 57.3%. Yellow dots per field of vision, as described by Bapu et al, was seen in all cases of AU depicting increased severity of disease [10].
Black dots (cadaverized hairs) and broken hairs correspond to the fragmented and destroyed hair shafts and represent a sign of disease activity. Black dots are only seen in dark-haired individuals. Inui et al [2], demonstrated BDs in 44.3% (133/300) cases of AA. Mane et al reported 67.7% (44/66) of patients had BDs [3] and Hegde et al [9] in 84% of cases. In our study BDs (Figures 3,5) were seen in 51 cases (70.8%).
Short vellus hairs (<10 mm) are a relatively common finding in AA (44.37%) [10]. They may be white and are most prevalent in the remitting stage of AA. Short vellus hairs may appear as circle hairs and is a predominant feature in some patients. Inui et al [2] observed SVHs in 72.7% (218/300) of cases. Mane et al [3] demonstrated SVHs in 40.9% of patients, Hegde et al [9] demonstrated SVHs in 68% of cases. In our study SVHs and curly hairs (CHs) (Figures 5, 6) were noted in 32 cases (44.44%).
Broken hairs are not exclusive to AA, as they are seen also in trichotillomania [1,3]. Inui et al [4] demonstrated BHs in 45.7% (137/300) of alopecia cases. Mane et al observed 55.4% patients with BH [3], and Hegde et al [9] reported the incidence of BH in 37.33% of cases. In our study BHs (Figure 3) was seen in 31 cases (43.05%).
Tapering hairs include exclamation mark hairs and coudability hairs and are a marker for disease activity and severity [4,11]. In our study THs were noted in 23 cases (31.9%). Inui et al noted THs in 31.7% (95/300) cases of AA [2], Mane et al [3] 12.1% (8/66), and Hegde et al [9] 14 (18.67%) cases of THs (Figure 3).
One patient with coexistent stable vitiligo was given bimatprost 0.03% ophthalmic solution, as there was presence of leukotrichia with the patch. In one study, after one patient was prescribed bimatoprost 0.03% ophthalmic solution for six weeks, there was partial repigmentation of the depigmented area, but the treatment was stopped due to localized hypertrichosis [12]. Another study showed partial repigmentation of leukotrichia [13]. White peripilar sign was also seen in 4 out of 7 cases on treatment with two to three hair units emerging from each follicl—a sign of hair regrowth. Although white peripilar has been described in other disorders to assess severity of disease, we found this to be a sign denoting hair growth.
Conclusion
YDS in increased number per field of vision is the most consistent finding and are seen with severe cases of AA, as they are in progressive AA and alopecia universalis. An increased number of SVH and terminal hairs were seen in patients who were being treated.
Footnotes
Funding: None.
Competing interests: The authors have no conflicts of interest to disclose.
All authors have contributed significantly to this publication.
References
- 1.Tosti A, Whiting D, Iorizzo M, et al. The role of scalp dermoscopy in the diagnosis of alopecia areata incognita. J Am Acad Dermatol. 2008;59:64–67. doi: 10.1016/j.jaad.2008.03.031. [DOI] [PubMed] [Google Scholar]
- 2.Inui S, Nakajima T, Nakagawa K, Itami S. Clinical significance of dermoscopy in alopecia areata: Analysis of 300 cases. Int J Dermatol. 2008;47:688–693. doi: 10.1111/j.1365-4632.2008.03692.x. [DOI] [PubMed] [Google Scholar]
- 3.Mane M, Nath AK, Thappa DM. Utility of dermoscopy in alopecia areata. Indian J Dermatol. 2011;56:407–411. doi: 10.4103/0019-5154.84768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Inui S, Nakajima T, Itami S. Coudability hairs: A revisited sign of alopecia areata assessed by trichoscopy. Clin Exp Dermatol. 2010;35:361–365. doi: 10.1111/j.1365-2230.2009.03510.x. [DOI] [PubMed] [Google Scholar]
- 5.Lallas A, Kyrgidis A, Tzellos TG, et al. Accuracy of dermoscopic criteria for the diagnosis of psoriasis, dermatitis, lichen planus and pityriasis rosea. Br J Dermatol. 2012;166:1198–205. doi: 10.1111/j.1365-2133.2012.10868.x. [DOI] [PubMed] [Google Scholar]
- 6.Tosti A. Dermoscopy of Hair and Scalp Disorders: With Clinical and Pathologic Correlations. London: Informa Healathcare; 2007. [Google Scholar]
- 7.Ihm CW, Hong SS, Mun JH, Kim HU. Histopathological pictures of the initial changes of the hair bulbs in alopecia areata. Am J Dermatopathol. 2004;26:249–253. doi: 10.1097/00000372-200406000-00017. [DOI] [PubMed] [Google Scholar]
- 8.Ross EK, Vincenzi C, Tosti A. Videodermoscopy in the evaluation of hair and scalp disorders. J Am Acad Dermatol. 2006;55:799–806. doi: 10.1016/j.jaad.2006.04.058. [DOI] [PubMed] [Google Scholar]
- 9.Hegde SP, Naveen KN, Athanikar SP, Reshme P. Clinical and dermatoscopic pattern of alopecia areata: A tertiary care centre experience. Int J Trichol. 2013;5:132–136. doi: 10.4103/0974-7753.125608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bapu NG, Chandrashekar L, Munisamy M, Thappa DM, Mohanan S. Dermoscopic findings of alopecia areata in dark skinned individuals: an analysis of 116 cases. Int J Trichol. 2014;6(4):156–159. doi: 10.4103/0974-7753.142853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shuster S. “Coudability”: a new physical sign of alopecia areata. Br J Dermatol. 1984;111:629. doi: 10.1111/j.1365-2133.1984.tb06637.x. [DOI] [PubMed] [Google Scholar]
- 12.Jha AK, Sinha R, Prasad S, Nandan N. Bimatoprost in periorbital vitiligo: a ray of hope or dilemma. J Eur Acad Dermatol Venereol. 2016;30:1247–1248. doi: 10.1111/jdv.13176. [DOI] [PubMed] [Google Scholar]
- 13.Jha AK, Sinha R. Bimatoprost in vitiligo. Clin Exp Dermatol. 2016;41:821–822. doi: 10.1111/ced.12904. [DOI] [PubMed] [Google Scholar]


