Abstract
Introduction
Pre-clinical studies suggest that angiotensin system inhibitors (ASI) and bevacizumab improve tumor perfusion and chemotherapy efficacy. We performed a retrospective study to examine whether concomitant ASI use during carboplatin and paclitaxel (CP) without or with bevacizumab (CPB) is associated with improved overall survival in patients with advanced non-squamous non-small cell lung cancer (NS-NSCLC).
Methods
In a retrospective cohort study, adult patients diagnosed with stage IIIB or IV NS-NSCLC between 2005 and 2011 were identified from tumor registries at one of four Kaiser Permanente regions. Survival differences between those who did and did not receive ASIs concomitant with chemotherapy (CP or CPB) were assessed using propensity score matched proportional hazard models. Overall survival (OS) was measured from the initiation of chemotherapy until death, disenrollment, or December 31, 2012.
Results
Of the 1,465 CP and 348 CPB patients included, 273 (19%) and 78 (22%), respectively, received concomitant ASI. For CP patients with and without concomitant ASI exposure, median overall survival (OS) was 12.0 and 8.4 months, respectively (crude hazard ratio (HR) 0.72, 95% confidence interval (CI) 0.63–0.84). For CPB patients comparable median OS was 14.9 and 11.9 months, respectively (crude HR 0.77, 95% CI 0.57–1.02). Using propensity score matched cohorts, the HR for concomitant ASI use was 0.73 (95% confidence interval (CI), 0.61–0.88), for CP patients and 0.79 (95% CI, 0.51–1.21) for CPB patients.
Conclusions
Concomitant ASI receipt during CP or CPB therapy for NS-NSCLC was associated with improved survival, though the association was only statistically significant in the CP group.
Keywords: Angiotensin, micro-environment, non-small cell lung cancer, bevacizumab, chemotherapy
Introduction
Non-small cell lung cancer (NSCLC) is the most common cause of cancer death in the world and is usually diagnosed at an incurable stage.1 Despite advances in immunotherapy and mutation driven sub-populations of advanced stage NSCLC, cytotoxic chemotherapy remains the cornerstone of initial treatment for the majority of advanced lung cancers. While individual patients with exceptional responses may derive meaningful benefit from chemotherapy, platinum-based doublet chemotherapy is characterized by moderate toxicity and modest efficacy with median survival after diagnosis in the 10-month range.2
In 2006, the FDA approved bevacizumab, a monoclonal antibody targeting vascular endothelial growth factor (VEGF), in combination with carboplatin and paclitaxel for the treatment of non-squamous NSCLC (NS-NSCLC). This approval was based on a randomized phase III study that demonstrated a 2-month improvement in median overall survival (OS).3 Multiple studies have sought to define a clinical or biomarker defined subgroup of patients who may derive greater clinical benefit from bevacizumab but, so far, without success.4
The efficacy of chemotherapy is dependent on drug delivery to the tumor,5 and drug delivery is in turn dependent on the adequacy of vascular structures supplying the tumor, the composition of the extracellular matrix, and the solid stress within the tumor.6,7 Collagen and hyaluronan are major structural components of the extracellular matrix that increase solid stress within tumors, resulting in compression of pliable blood vessels.8 In murine models, losartan, a generic angiotensin receptor blocker (ARB) used to treat high blood pressure, decreases intra-tumor collagen, hyaluronan, and solid stress while improving chemotherapy delivery and efficacy.9 Several small retrospective studies have identified an association of angiotensin system inhibitors (ASIs), which include angiotensin-converting-enzyme inhibitors (ACEIs) and angiotensin receptor blockers (ARBs), with improved survival in patients with advanced stage malignancies, including NSCLC,10 pancreatic cancer,11 gastric cancer,12 and renal cell carcinoma.13 No studies to date have examined the receipt of ASIs concurrently with bevacizumab-containing chemotherapy, where ASIs and bevacizumab may both work together to improve tumor perfusion.
In exploratory analyses, we examined the association between concomitant ASI receipt and overall survival (OS) in a cohort of advanced stage NS-NSCLC receiving chemotherapy with carboplatin and paclitaxel without (CP) or with bevacizumab (CPB). Our primary aim was to assess whether receipt of an ASI concurrently with chemotherapy was associated with improved survival. We were also interested in whether use with bevacizumab resulted in synergistic improvements in chemotherapy efficacy and OS.
Materials and Methods
Research Setting
Patients included in this study received their medical care from Kaiser Permanente (KP), a large fully-integrated non-profit health care delivery system, in one of four regions: Colorado, Northwest, Northern California, and Southern California. The majority of cancer care was delivered by Permanente medical group physicians who exclusively treat Kaiser Permanente health plan members. This study was approved by the Institutional Review Boards of the four participating institutions.
Data Sources
Data were extracted from the four KP regions’ Virtual Data Warehouse (VDW). As described elsewhere, the VDW is a standardized data model that was developed for research use across the Cancer Research Network (CRN).14–16 The VDW Tumor Registry (VTR) dataset contains data that conform with standards of the North American Association of Central Cancer Registries standards17 and the National Cancer Institute’s Surveillance, Epidemiology and End Results program. The VTR data on diagnosis and staging are derived from manual reviews of cancer patients’ medical charts by trained tumor registrars. The VTR data also include date of diagnosis, first-course definitive treatment (i.e., surgery, radiotherapy, chemotherapy, etc.), and tumor and patient demographic characteristics. The VDW Pharmacy and Infusion datasets capture National Drug Code (NDC)-based prescription drug products dispensed from outpatient and infusion center pharmacies, respectively. Data in the VDW pertaining to deaths are derived from the tumor registries, membership data, state-level (CA, CO, OR, WA) mortality files, and Social Security Administration data. All analyses were performed using SAS 9.2 (SAS Inc., Cary, NC).
Study Sample
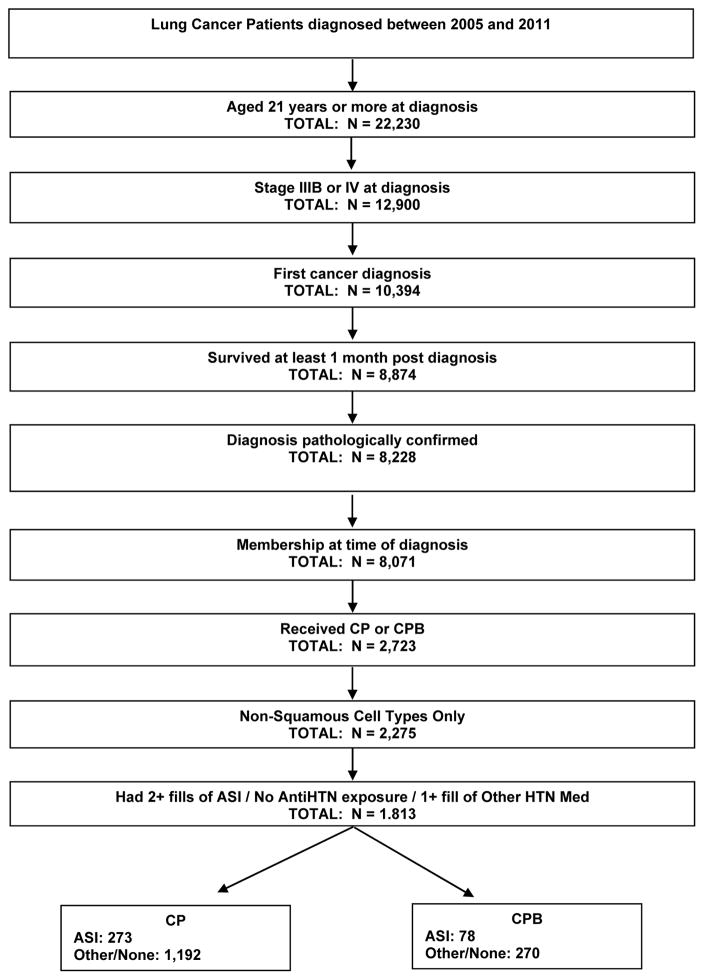
Data from patients aged ≥21 years as of date of stage IIIB/IV non-squamous NSCLC diagnosis between January 1, 2005, and December 31, 2011, were extracted from the VTR. Eligibility was limited to patients: (1) with their first cancer diagnosis; (2) enrolled in a KP health plan at the time of cancer diagnosis; (3) survived at least one month after cancer diagnosis; (4) received either CP or CPB first-line chemotherapy; and (5) had a pathologically confirmed diagnosis.
Medication Exposures
While we primarily were interested in ASI receipt (either an ACEI or ARB), we also collected information on other commonly used hypertension (HTN) medications including beta blockers (BBs), calcium channel blockers (CCBs), diuretics, alpha blockers, and vasodilators. ASI exposure was defined as two purchases of the medication within a timeframe of 90 days before to 90 days after the start of chemotherapy. We examined several exposure models, and identified this model as providing the most assurance that a patient was likely to be receiving an ASI for the 12–18 weeks when a patient was receiving first-line chemotherapy. Primary analyses compared “ASI” patients, who were receiving an ACEI or ARB with or without additional HTN medications, versus “other” patients, who could have been receiving any non-ASI HTN medications, or no HTN medications. Because patient care was delivered within an integrated health system with a preferred medication formulary, lisinopril and losartan comprised more than 95% of the ACEIs and ARBs purchased, respectively.
Identification of First-line Carboplatin, Paclitaxel, and Bevacizumab
Eligible patients receiving first-line CP or CPB were identified using the VDW Pharmacy, Procedure, and Infusion datasets with methods described previously.14,18–20 First-line chemotherapy initiation was defined as the date of the first chemotherapy regimen initiated after cancer diagnosis.
Baseline Characteristics
Figure 1 shows the selection of patients with NSCLC from which the study cohort was derived. The study cohort consisted of 1,813 participants of whom 1,465 (78%) received CP chemotherapy and 348 (22%) received CPB chemotherapy. Of the patients receiving CP or CPB, 273 (19%) and 78 (22%), respectively, received an ASI concomitantly. We considered 13 baseline characteristics that are described in Table 1. Both CP and CPB patients who received an ASI (vs. did not receive) had greater comorbidity burden (cardiovascular disease, diabetes, PVD, other diseases; all p < 0.01), and were significantly more likely to have received other antihypertensive medications during the treatment period. In addition, CP patients who received an ASI were more like to be older (p < 0.01).
Figure 1.
Selection of Patients for NSCLC Cohort
Table 1.
Baseline characteristics of patients with advanced non-squamous NSCLS receiving carboplatinum and paclitaxel (CP) or carboplatinum, paclitaxel, bevacizumab (CPB)
| CP, Pre Matched | CP, Post Matched | CPB, Pre Matched | CPB, Post Matched | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Characteristic | ASI (n=273) | Other (n=1192) | p-value | ASI (n=255) | Other (n=255) | p-value | ASI (n=78) | Othern= (270) | p-value | ASI (n=61) | Other (n=61) | p-value |
| Demographics, N (%) | ||||||||||||
| Age at diagnosis, Mean (std) | 68 (8) | 62 (10) | < 0.01 | 67 (8) | 67 (9) | 0.76 | 63 (8) | 59 (11) | < 0.01 | 62 (8) | 62 (11) | 0.93 |
| Non-White | 90 (33) | 381 (32) | 0.75 | 83 (33) | 77(30) | 0.63 | 23 (30) | 91 (34) | 0.75 | 18 (30) | 14 (23) | 0.40 |
| Male | 139 (51) | 621 (52) | 0.72 | 129 (51) | 128 (50) | 0.66 | 38 (49) | 134 (50) | 0.72 | 27 (44) | 29 (47) | 0.99 |
| Poor/Undifferentiated/Unk Tumor Grade | 55 (71) | 225 (83) | 0.01 | 223 (87) | 222 (87) | 0.99 | 55 (71) | 225 (83) | 0.01 | 44 (72) | 49 (80) | 0.09 |
| Education | 0.01 | 0.91 | 0.60 | 0.22 | ||||||||
| Rank 1, lowest | 61 (22) | 202 (17) | 54 (21) | 29 (11) | 18 (23) | 48 (18) | 7 (11) | 7 (11) | ||||
| Rank 2 | 46 (17) | 241 (20) | 44 (17) | 45 (18) | 10 (13) | 41 (15) | 11 (18) | 22 (36) | ||||
| Rank 3 | 46 (17) | 252 (21) | 45 (18) | 47 (18) | 14 (18) | 62 (23) | 14 (23) | 9 (15) | ||||
| Rank 4 | 73 (27) | 242 (20) | 68 (27) | 70 (27) | 18 (23) | 49 (18) | 14 (23) | 14 (23) | ||||
| Rank 5, highest | 47 (17) | 255 (21) | 44 (17) | 46 (18) | 18 (23) | 70 (26) | 15 (25) | 9 (15) | ||||
| Risk Factors | ||||||||||||
| COPD | 111 (41) | 315 (26) | 0.27 | 135 (53) | 137 (54) | 0.72 | 25 (32) | 124 (46) | 0.57 | 25 (41) | 29 (48) | 0.36 |
| Cardiovascular Disease1 | 124 (45) | 253 (21) | < 0.01 | 108 (42) | 109 (43) | 0.86 | 25 (32) | 38 (14) | < 0.01 | 15 (25) | 17(28) | 0.99 |
| Peripheral Vascular Disease | 88 (32) | 172 (14) | < 0.01 | 74 (29) | 81 (32) | 0.77 | 21 (27) | 26 (10) | < 0.01 | 19 (31) | 11 (18) | 0.06 |
| Diabetes | 111 (41) | 141 (12) | < 0.01 | 93 (36) | 94 (37) | 0.71 | 27 (35) | 16 (6) | < 0.01 | 14 (23) | 14 (23) | 0.83 |
| Other Disease2 | 111 (41) | 315 (26) | < 0.01 | 101 (40) | 106 (42) | 0.52 | 41 (53) | 68 (25) | < 0.01 | 30 (49) | 15 (25) | < 0.01 |
| Health Plan | 0.03 | 0.67 | 0.10 | 0.18 | ||||||||
| Site(s) 2 | 119 (44) | 432 (36) | 107 (42) | 99 (39) | 40 (51) | 106 (39) | 29 (48) | 32 (54) | ||||
| Site(s) 3 | 43 (16) | 173 (15) | 40 (16) | 40 (16) | 11 (14) | 62 (23) | 9 (15) | 11 (18) | ||||
| Other HTN Med | 126 (46) | 320 (27) | < 0.01 | 112 (44) | 108 (42) | 0.59 | 55 (71) | 86 (32) | 27 (44) | 26 (43) | 0.72 | |
| Smoking Status | 0.78 | 0.86 | 0.21 | 0.83 | ||||||||
| Ever | 177 (65) | 748 (63) | 167 (65) | 163 (64) | 54 (69) | 163 (60) | 44 (72) | 38 (62) | ||||
| Never | 40 (15) | 192 (16) | 37 (15) | 37 (15) | 13 (17) | 71 (26) | 10 (16) | 13 (21) | ||||
| Unknown | 56 (21) | 252 (21) | 51 (20) | 55 (22) | 11 (14) | 36 (33) | 7 (11) | 10 (16) | ||||
| Weight Change 6 mos prior to Ca Diagnosis | 0.12 | 0.72 | 0.44 | 0.97 | ||||||||
| > 6% weight loss | 56 (21) | 205 (17) | 52 (20) | 49 (19) | 16 (21) | 40 (15) | 12 (20) | 12 (20) | ||||
| <= 6% weight loss or weight gain | 128 (47) | 522 (44) | 119 (47) | 117 (46) | 40 (51) | 141 952) | 32 (52) | 30 (49) | ||||
| Unknown/Missing | 89 (33) | 465 (39) | 84 (33) | 89 (35) | 22 (28) | 89 (33) | 17 (28) | 19 (31) | ||||
| Hypertension Meds | NA | NA | NA | NA | ||||||||
| ACEI | 210 (77) | -- | -- | 199 (78) | -- | -- | 55 (71) | -- | -- | 45 (74) | -- | -- |
| ARB | 63 (23) | -- | -- | 56 (22) | -- | -- | 23 (29) | -- | -- | 16 (26) | -- | -- |
Cardiovascular Disease includes diagnoses of cardiovascular disease, congestive heart failure and myocardial infarction
Other Disease includes liver disease, plegia, renal disease, peptic ulcer disease, and rheumatic disease
Propensity Score Matching
To adjust for potential confounding factors and minimize bias due to the patients’ non-random assignment to ASI receipt and non-receipt, CP and CPB patients were matched separately using propensity scores calculated by logistic regression using the baseline characteristics described above (Supplemental see Table 1). A greedy nearest-neighbor matching algorithm with a caliper width equal to 0.2 of the standard deviation of the logit of the propensity score was used to create matching pairs. The balance of the matched model was assessed using standardized differences of the means for each covariate. A threshold of 0.10 was used to indicate imbalance in the baseline covariates.21–30 Refinements of the matching model were made iteratively until balance was achieved. Final covariates included in CP propensity score were age at diagnosis, race/ethnicity, sex, education (census data proxies ranked into quintiles), chronic obstructive pulmonary disease (COPD), cardiovascular disease (includes diagnoses of cardiovascular disease, congestive heart failure and myocardial infarction), peripheral vascular disease (PVD), diabetes, other comorbid diseases (including liver disease, plegia, renal disease, peptic ulcer disease, and rheumatic disease), health plan site, and other anti-hypertensive medications (defined as a dispense of any other [i.e., non-ASI] anti-hypertensive medication during the study period). Final covariates included in the CPB propensity score were age at diagnosis, sex, cardiovascular disease, diabetes and other antihypertensive medications.
Two hundred fifty-five (93.4%) of the 273 ASI patients were matched to a non-ASI patient with a similar propensity score in the CP group while 61 (78.2%) of the 78 ASI patients were matched to a non-ASI patient with a similar propensity score in the CPB group. Balance before and after propensity score matching is summarized in Supplemental see Table 2 and Supplemental see Figures 1 and 2. In the matched sample, standardized differences of the means ranged from 0.005 to 0.064 in the CP group and 0.015 to 0.075 in the CPB group. In examining the standardized differences and associated graphs, we observed that differences between the treatment groups were reduced by the matching.
To address potential biases because of incomplete matching, we constructed a second matched sample using optimal matching. In the matched sample constructed using optimal matching, the largest absolute standardized difference of the means in the CP group was 0.099 (cardiovascular disease) with the remaining standardized differences all less than 0.070. The largest absolute standardized difference of the means in the CPB was other antihypertensive medications (0.050) with the remaining standardized differences all less than 0.050 (data not shown).
Survival Analysis
Overall survival, defined as the months from first-line chemotherapy initiation until death from any cause, was the main outcome measure. Patients who dis-enrolled from the health plan or were alive at the end of the study period (December 31, 2012) were censored on those dates. We examined OS separately for patients who received either CP or CPB. Within the CP or CPB patient groups, we compared those with and without ASI receipt concomitant with chemotherapy administration. Medians and interquartile ranges of time to death were estimated using the Kaplan-Meier method and compared between groups with log-rank tests.31 Using Cox regression modeling with a robust sandwich variance estimator on the matched sample pairs, we estimated relative hazard ratios (HRs) and their 95% confidence intervals (CIs) to evaluate the effect of ASI receipt on OS.21–30
Results
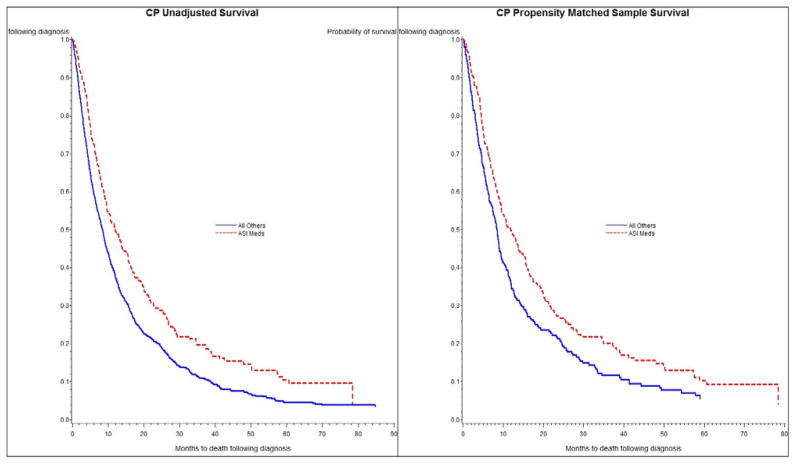
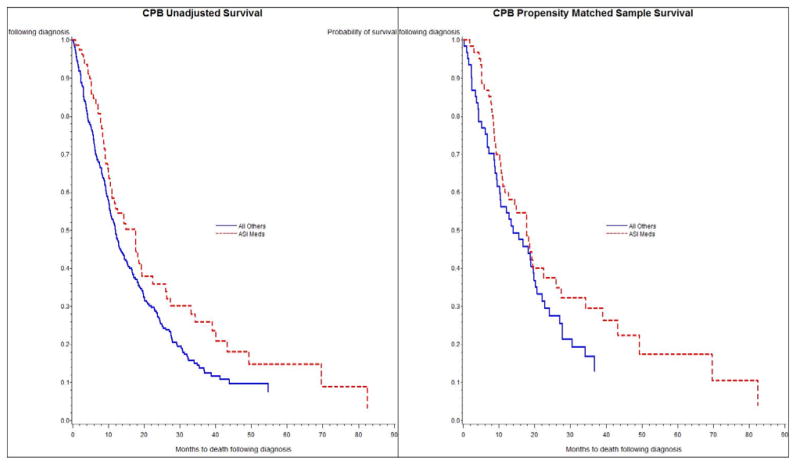
For patients who received CP with vs. without concomitant ASI receipt, median OS was 12.0 and 8.4 months, respectively (crude HR 0.72, 95% confidence interval (CI) 0.63–0.84; Table 2). For patients who received CPB with vs. without concomitant ASI receipt, median OS was 14.9 and 11.9 months, respectively (crude HR 0.77, 95% CI 0.57–1.02; Table 2). Figures 2 and 3 contain the Kaplan-Meier crude survival curves. Statistically, the crude survival curves are significantly different for the CP group (figure 2a; log-rank test; p < 0.01), but not for the CPB group (figure 3a. log-rank test; p = 0.07). Crude median OS for the patients who received CP or CPB were 8.9 and 12.3 months, respectively.
Table 2.
Cox Proportional Hazard Models for Patients with Advanced Non-squamous NSCLC Receiving Carbloplatinum and Paclitaxel (CP) or Carboplatinum, Paclitaxel, and Bevacizumab (CPB) taking an ASI compared to patients not on an ASI (referent)
| Analysis | CP | CPB | ||
|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | |
| Unadjusted Survival1 | 0.72 | 0.63–0.84 | 0.77 | 0.57–1.02 |
| Matched Analysis using 0.20 Caliper2 | 0.73 | 0.61–0.88 | 0.79 | 0.52–1.21 |
| Optimal Matched Analysis3 | 0.80 | 0.67–0.97 | 0.70 | 0.49–1.01 |
P-value based on the log-rank test
N = 255 matched pairs in the CP group and N = 61 pairs in the CPB group
CP: N = 273 for ASI, N = 259 for Other; CPB: N = 78 for ASI, N = 74 for Other
Figure 2.
Figure 2a and 2b. Unadjusted (2a) and Propensity Score Matched (2b) Kaplan-Meier Survival Curves for Patients with Non-Squamous, Non-Small-Cell Lung Cancer Receiving Carboplatin and Paclitaxel (CP) with or without and Concomitant Receipt of Angiotensin-System Inhibitors (ASIS) with rr without Concomitant Receipt of Angiotensin-System Inhibitors (ASIS)
Figure 3.
Figure 3a and 3b. Unadjusted (3a) and Propensity Score Matched (3b) Kaplan-Meier Survival Curves for Patients with Non-Squamous Non-Small-Cell Lung Cancer Receiving Carboplatin, Paclitaxel and Bevacizumab (CPB) with or without Concomitant Receipt of Angiotensin-System Inhibitors (ASIS).
For CP patients with concomitant ASI receipt, the HRs derived from the caliper matched model similarly demonstrated statistically significant OS benefit, (HR 0.73, 95% CI 0.61–0.88) relative to patients with no ASI receipt (Table 2). For the smaller CPB patient group, the HR was <1 but did not reach statistical significance. Adjusted survival curves from the caliper matched pair analysis are shown in Figures 2b and 3b.
Similar results were obtained in the sub-analysis using the optimal matched sample. Statistically significant OS benefit was observed in the CP group (HR 0.80, 95% CI 0.67–0.97) for patients with concomitant ASI while statistical significance was not reached in the CPB group (HR 0.70, 95% CI 0.49–1.01: Table 2).
Conclusion
In this exploratory retrospective cohort study, we compared OS differences between patients who did or did not receive an ASI during first-line CP or CPB chemotherapy for advanced non-squamous NSCLC. We observed a numerically increased OS with ASI receipt concomitant with chemotherapy in patients undergoing either regimen, though this association was only significant in the CP group. This increased OS remained after adjustment for multiple factors, including comorbid conditions that may influence use of ASIs and the use of other anti-hypertensive medications. These findings support pre-clinical evidence that inhibition of the angiotensin II receptor improves tumor perfusion and chemotherapy delivery,9 and mirror prior studies that have shown an association between ASI receipt and improved OS for patients receiving treatment for advanced solid tumors10–13
Separately, we were interested in whether ASI receipt may be an unrecognized predictor of OS benefit from bevacizumab-based chemotherapy due to synergistic effects of these two agents on the tumor microenvironment. Although we did observe somewhat improved OS for ASI receipt during CPB chemotherapy, the magnitude of the benefit from ASI receipt was similar to the CP cohort. This suggests that ASIs and bevacizumab did not have a synergistic benefit on OS in our study. While several prior studies have shown a lack of benefit for bevacizumab in older patient populations,32,33 the small sample sizes in our CPB group precluded additional subgroup analyses in those under 65-year-olds where synergistic OS benefit might have been concentrated.
One possible explanation for our observed OS benefit of ASI receipt may be that an NSCLC patient who is still “fit” enough to require anti-hypertension medication and adherent enough to purchase it may be more likely to have a better performance status, higher body mass index,34 and better medical compliance--factors that could be associated with improved survival in NSCLC. If this were the main explanation for the benefit observed for ASI receipt, one would also expect that patients who received non-ASI anti-hypertension medications would have similar OS benefit. However, we adjusted for concomitant non-ASI anti-hypertensive medication exposure and found no decrease in the observed OS benefit.
While some anti-hypertension medications are prescribed for patients on therapeutic VEGF inhibition, the vast majority of anti-hypertensive prescriptions were prescribed before cancer diagnosis for comorbid hypertension. Because the possible role for anti-hypertension medications in cancer treatment is not widely recognized, we believe that anti-hypertension medications used during this study period represent a relatively “random” treatment allocation compared to treatments received as part of a patient’s cancer care, strengthening the validity of our observed survival benefit with concomitant ASI use.
The main strengths of our study include its relatively large sample size and use of high-quality exposure and outcome data from validated registries and datasets. Additionally, KP’s population is socioeconomically and racially/ethnically diverse. Although it may limit generalizability to healthcare delivered outside Kaiser Permanente healthcare system, it is noted that nearly all covered members have access to primary care and outpatient pharmacy benefits that subsidize prescriptions for chronic comorbid conditions. This increases the chances that patients who needed antihypertensive medications were receiving them.
The primary limitation of our study is its observational nature in which we are unlikely to have accounted for all factors that could influence the association of ASI use with OS.35 For example, we do not have a good measure of performance status,36 which is available in clinical trial settings but not in routine clinical care. We also did not consider some factors that are may impact OS, including use of other medications such as metformin,37 or statins.38 However, we did consider comorbid conditions like diabetes and cardiovascular disease that would normally be associated with the use of those medications, and these did not influence the overall association of concomitant ASI use with OS, suggesting that, as with use of non-ASI anti-hypertensive medications, these factors are unlikely to modify the observed associations.
We were also unable to directly confirm the ingestion of ASIs, relying instead on prescription purchases. It is possible that some patients in our ASI group stopped or held their ASI before chemotherapy due to weight loss, dropping blood pressure, or perceived lack of value. However, by defining ASI exposure as at least two prescription purchases within the 180-day window centered on date of initiation of chemotherapy, we feel confident that a large majority of ASI patients in our study were actually using their ASI during chemotherapy.
Many patients who present with NSCLC have co-morbid illnesses for which they are receiving anti-hypertension medications. While such medications are typically thought of as inert bystanders in the oncology clinic, some of these medications may influence the host or tumor in ways that affect treatment efficacy and subsequent survival. Our study provides evidence that ASIs could represent an important augmentative anti-cancer therapy that has been hidden in plain sight. While many novel anti-cancer therapies approved in recent years have targeted smaller populations of patients with costs in excess of $100,000 per year, generic ASIs have the potential to meaningfully improve treatment efficacy across a broad spectrum of malignancies for less than $100 per year.
In summary, we observed an association between ASI receipt during CP chemotherapy and improved overall survival. We observed a similar, albeit non-significant, association between ASI receipt and CPB chemotherapy; however, the smaller sample size for this group of provides less confidence in these results. Further studies are warranted to confirm our findings.
Supplementary Material
Clinical Practice Points.
ASIs are commonly taken during chemotherapy for comorbid hypertension or cardiovascular disease.
Several small retrospective studies have already shown an association between ASI receipt and improved survival.
In our retrospective study, which is the largest of it’s kind and included patients taking bevacizumab for the first time, despite older age and greater comorbidity burden, receipt of ASI was associated with improved overall survival.
For patients experiencing HTN requiring treatment during bevacizumab therapy, ASIs may be a preferred class of anti-hypertensive agent in patients with good renal function.
Future prospective and retrospective clinical trials in NSCLC should track ASI use as a potentially pertinent clinical characteristic that could effect study outcomes.
Further study of the effect of ASIs during treatment for NSCLC is warranted.
Acknowledgments
Author Contributions: Ritzwoller, and Carroll had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Menter, Ritzwoller, Hornbrook, Sakoda, Kushi, Quinn.
Acquisition of data: Carroll, Hornbrook, Kushi, Quinn, Ritzwoller, Sakoda.
Analysis and interpretation of data: Menter, Ritzwoller, Carroll, Delate, Hornbrook, Sakoda, Kushi, Quinn.
Drafting of the manuscript: Menter.
Critical revision of the manuscript for important intellectual content: Menter, Ritzwoller, Delate, Carroll, Hornbrook, Sakoda, Kushi, Quinn, Jain.
Statistical analysis: Carroll, Ritzwoller.
Obtained funding: Menter, Ritzwoller, Hornbrook, Kushi, Quinn.
Study supervision: Menter, Ritzwoller.
Funding/Support: Funding for this research was provided by the Kaiser Permanente Center for Effectiveness and Safety Research (CESR, Elizabeth McGlynn, Director), the National Cancer Institute Grant No. U24 CA171524 (L. Kushi, PI), and the National Cancer Institute Grant No. PO1 CA080124 (R. Jain, PI).
Role of the Sponsors: The funding agencies had no role in the design and conduct of the study, in the collection, analysis, and interpretation of the data, or in the preparation, review, or approval of the manuscript.
Additional Contributions: We thank John Adams, PhD, for biostatistical consultation and Michelle Wrenn for organizational support. We also thank Valerie S. Lee, Julie W. Liu, and Kristal C. Rust for programming and data extraction support.
Funding/Support:
Funding for this research was provided by the Kaiser Permanente Center for Effectiveness and Safety Research (CESR) and the National Cancer Institute (Grant No. U24 CA171524).
Footnotes
Conflict of Interest
Financial Disclosures: Menter, Ritzwoller, Delate, Carroll, Hornbrook, Sakoda, Kushi, Quinn—None reported
Rakesh Jain co-founded, has equity in, and sits on the board of directors of XTuit, a company that is developing anticancer therapies. XTuit holds a license for a patent filed by Massachusetts General Hospital on matrix-depleting drugs based on work by Jain and his colleagues. Jain had access only to de-identified summary data.
PRIOR PRESENTATIONS: 2014 ASCO Annual Meeting, Chicago, Lung Cancer Poster Session
References
- 1.Brambilla E, Travis WD, Stewart BW, Wild CP, editors. Pathology & Genetics: Tumours of the lung, pleura, thymus and heart. [Accessed 02/29/2016];World Health Organization Classification of Tumors. 2014 Available from: http://www.iarc.fr/en/publications/pdfs-online/pat-gen/bb10/BB10.pdf.
- 2.NSCLC Meta-Analyses Collaborative Group. Chemotherapy in addition to supportive care improves survival in advanced non-small-cell lung cancer: a systematic review and meta-analysis of individual patient data from 16 randomized controlled trials. J Clin Oncol. 2008;26(28):4617–4625. doi: 10.1200/JCO.2008.17.7162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355(24):2542–2550. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 4.Jain RK. Antiangiogenesis strategies revisited: from starving tumors to alleviating hypoxia. Cancer Cell. 2014;26(5):605–622. doi: 10.1016/j.ccell.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim ES, Lee JJ, He G, et al. Tissue platinum concentration and tumor response in non-small-cell lung cancer. J Clin Oncol. 2012;30(27):3345–3352. doi: 10.1200/JCO.2011.40.8120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jain RK. Normalizing tumor microenvironment to treat cancer: bench to bedside to biomarkers. J Clin Oncol. 2013;31(17):2205–2218. doi: 10.1200/JCO.2012.46.3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Minchinton AI, Tannock IF. Drug penetration in solid tumours. Nat Rev Cancer. Cancer. 2006;6(8):583–592. doi: 10.1038/nrc1893. [DOI] [PubMed] [Google Scholar]
- 8.Stylianopoulos T, Martin JD, Chauhan VP, et al. Causes, consequences, and remedies for growth-induced solid stress in murine and human tumors. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(38):15101–15108. doi: 10.1073/pnas.1213353109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chauhan VP, Martin JD, Liu H, et al. Angiotensin inhibition enhances drug delivery and potentiates chemotherapy by decompressing tumour blood vessels. Nat Commun. 2013;4:2516. doi: 10.1038/ncomms3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilop S, von Hobe S, Crysandt M, Esser A, Osieka R, Jost E. Impact of angiotensin I converting enzyme inhibitors and angiotensin II type 1 receptor blockers on survival in patients with advanced non-small-cell lung cancer undergoing first-line platinum-based chemotherapy. J Cancer Res Clin Oncol. 2009;135(10):1429–1435. doi: 10.1007/s00432-009-0587-3. [DOI] [PubMed] [Google Scholar]
- 11.Nakai Y, Isayama H, Ijichi H, et al. Inhibition of renin-angiotensin system affects prognosis of advanced pancreatic cancer receiving gemcitabine. Br J Cancer. 2010;103(11):1644–1648. doi: 10.1038/sj.bjc.6605955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim ST, Park KH, Oh SC, et al. How does inhibition of the renin-angiotensin system affect the prognosis of advanced gastric cancer patients receiving platinum-based chemotherapy? Oncology. 2012;83(6):354–360. doi: 10.1159/000337979. [DOI] [PubMed] [Google Scholar]
- 13.Keizman D, Huang P, Eisenberger MA, et al. Angiotensin system inhibitors and outcome of sunitinib treatment in patients with metastatic renal cell carcinoma: a retrospective examination. Eur J Cancer. 2011;47(13):1955–1961. doi: 10.1016/j.ejca.2011.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ritzwoller DP, Carroll NM, Delate T, et al. Patterns and predictors of first-line chemotherapy use among adults with advanced non-small cell lung cancer in the cancer research network. Lung Cancer. 2012;78(3):245–252. doi: 10.1016/j.lungcan.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ritzwoller DP, Carroll N, Delate T, et al. Validation of electronic data on chemotherapy and hormone therapy use in HMOs. Med Care. 2013;51(10):e67–73. doi: 10.1097/MLR.0b013e31824def85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ross TR, Ng DJ, Brown JS, et al. The HMO Research Network Virtual Data Warehouse: a public data model to support collaboration. eGEMS. 2014;2(1):Article 2. doi: 10.13063/2327-9214.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.North American Association of Central Cancer Registries. [Accessed 02/29/2016];NAACCR Strategic Management Plan 2011–2016. 2011 Availaboe from: www.naaccr.org/AboutNAACCR/SMP.aspx.
- 18.Delate T, Won K, Carroll N, et al. Factors associated with first-line bevacizumab use in advanced non-squamous non-small cell lung cancer. J Cancer Res Ther. 2014 Jan;2(1):1–8. [Google Scholar]
- 19.Ritzwoller DP, Carroll NM, Delate T, et al. Comparative effectiveness of adjunctive bevacizumab for advanced lung cancer: the cancer research network experience. J Thorac Oncol. 2014;9(5):692–701. doi: 10.1097/JTO.0000000000000127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Delate T, Bowles EJ, Pardee R, et al. Validity of Eight Integrated Healthcare Delivery Organizations’ Administrative Clinical Data to Capture Breast Cancer Chemotherapy Exposure. Cancer Epidemiol Biomarkers Prev. 2012 Apr;21(4):637–680. doi: 10.1158/1055-9965.EPI-11-1075. Epub 2012 Feb 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.D’Agostino RB., Jr Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med. 1998;17(19):2265–2281. doi: 10.1002/(sici)1097-0258(19981015)17:19<2265::aid-sim918>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 22.Austin PC. An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivariate Behav Res. 2011;46(3):399–424. doi: 10.1080/00273171.2011.568786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hershman DL, Wright JD. Comparative effectiveness research in oncology methodology: observational data. J Clin Oncol. 2012;30(34):4215–4222. doi: 10.1200/JCO.2012.41.6701. [DOI] [PubMed] [Google Scholar]
- 24.Faires DE, Leon AC, Haro JM, Obenchain RL, editors. Analysis of observational health care data using SAS. 1. Cary, NC: SAS Institute, Inc; 2010. Propensity score stratification and regression; pp. 23–46. [Google Scholar]
- 25.Robins JM, Hernan MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology. 2000;11(5):550–560. doi: 10.1097/00001648-200009000-00011. [DOI] [PubMed] [Google Scholar]
- 26.Kurth T, Walker AM, Glynn RJ, et al. Results of multivariable logistic regression, propensity matching, propensity adjustment, and propensity-based weighting under conditions of nonuniform effect. Am J Epidemiol. 2006;163(3):262–270. doi: 10.1093/aje/kwj047. [DOI] [PubMed] [Google Scholar]
- 27.Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009;28(25):3083–3107. doi: 10.1002/sim.3697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Austin PC, Mamdani MM. A comparison of propensity score methods: a case-study estimating the effectiveness of post-AMI statin use. Stat Med. 2006;25(12):2084–2106. doi: 10.1002/sim.2328. [DOI] [PubMed] [Google Scholar]
- 29.Austin PC. The use of propensity score methods with survival or time-to-event outcomes: reporting measures of effect similar to those used in randomized experiments. Stat Med. 2014;33(7):1242–1258. doi: 10.1002/sim.5984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sperry SM, Charlton ME, Pagedar NA. Association of sentinel lymph node biopsy with survival for head and neck melanoma: survival analysis using the SEER database. JAMA Otolaryngol Head Neck Surg. 2014;140(12):1101–1109. doi: 10.1001/jamaoto.2014.2530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cox DR. Partial likelihood. Biometrika. 1975;62:269–276. [Google Scholar]
- 32.Ramalingam SS, Dahlberg SE, Langer CJ, et al. Outcomes for elderly, advanced-stage non small-cell lung cancer patients treated with bevacizumab in combination with carboplatin and paclitaxel: analysis of Eastern Cooperative Oncology Group Trial 4599. J Clin Oncol. 2008;26(1):60–65. doi: 10.1200/JCO.2007.13.1144. [DOI] [PubMed] [Google Scholar]
- 33.Zhu J, Sharma DB, Gray SW, Chen AB, Weeks JC, Schrag D. Carboplatin and paclitaxel with vs without bevacizumab in older patients with advanced non-small cell lung cancer. JAMA. 2012;307(15):1593–1601. doi: 10.1001/jama.2012.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dahlberg SE, Schiller JH, Bonomi PB, et al. Body mass index and its association with clinical outcomes for advanced non-small-cell lung cancer patients enrolled on Eastern Cooperative Oncology Group clinical trials. J Thorac Oncol. 2013;8(9):1121–1127. doi: 10.1097/JTO.0b013e31829cf942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bosco JL, Silliman RA, Thwin SS, et al. A most stubborn bias: no adjustment method fully resolves confounding by indication in observational studies. J Clin Epidemiol. 2010;63(1):64–74. doi: 10.1016/j.jclinepi.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hoang T, Dahlberg SE, Sandler AB, Brahmer JR, Schiller JH, Johnson DH. Prognostic models to predict survival in non-small-cell lung cancer patients treated with first-line paclitaxel and carboplatin with or without bevacizumab. J Thorac Oncol. 2012;7(9):1361–1368. doi: 10.1097/JTO.0b013e318260e106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lin JJ, Gallagher EJ, Sigel K, et al. Survival of patients with stage IV lung cancer with diabetes treated with metformin. Am J Respir Crit Care Med. 2015;191(4):448–454. doi: 10.1164/rccm.201407-1395OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saxena A, Becker D, Preeshagul I, Lee K, Katz E, Levy B. Therapeutic Effects of Repurposed Therapies in Non-Small Cell Lung Cancer: What Is Old Is New Again. Oncologist. 2015;20(8):934–945. doi: 10.1634/theoncologist.2015-0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.