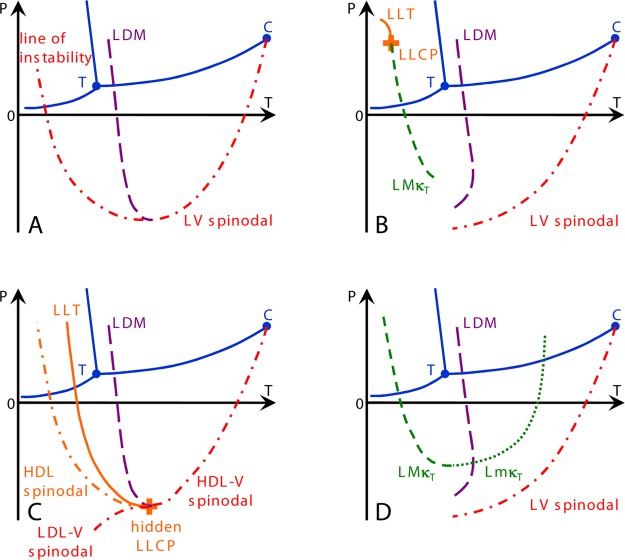
Figure 3.
Scenarios that might account for the behavior observed in Figure 1. (A) Speedy’s stability limit conjecture,33 (B) Poole et al.’s second critical point,23 (C) Poole et al.’s “weak bond”-modified van der Waals model, now the critical-point-free scenario,46 and (D) Sastry et al.’s singularity-free scenario.31 Continuous blue curves show the known equilibrium coexistence lines between liquid, solid, and vapor with the triple point marked as T. Liquid–vapor equilibrium terminates at the critical point C. The long-dashed purple line shows the line of density maxima (LDM), and the short-dashed and dotted green lines are the lines of isothermal compressibility maxima (LMκT) and minima (LmκT), respectively. Dash–dotted lines indicate lines of instability. In scenarios A and C, the LDM keeps a negative slope and ends at a line of instability. In scenarios B and D, the LDM reaches a maximum temperature and changes its slope, eventually merging with a line of density minima (not shown for clarity). When the scenario comprises a liquid–liquid transition, it is displayed with a continuous orange line (LLT), and the liquid–liquid critical point is shown as an orange plus. Adapted from ref (47). Copyright 2014 National Academy of Sciences.