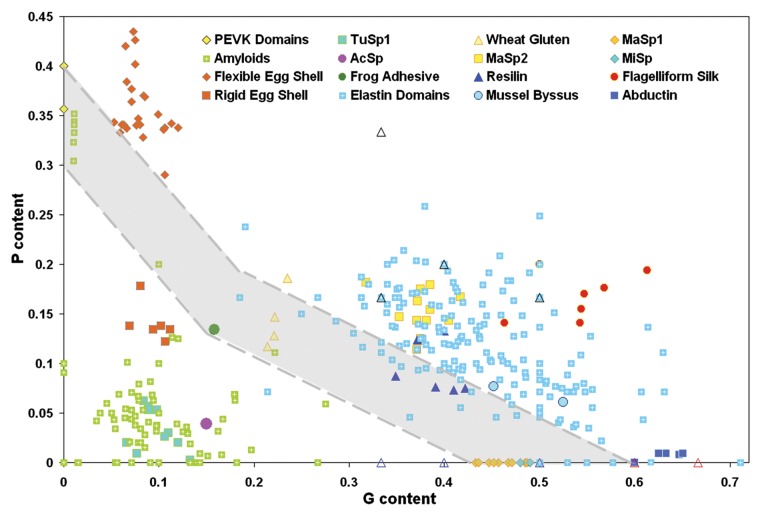
Figure 3. A two-dimensional plot correlating proline and glycine content for a wide variety of elastomeric and amyloidogenic peptides. Elastomeric proteins are characterized by high GP content and are located in the upper-right part of this plot. Contrarily, amyloidogenic peptides are characterized by low PG content and therefore are located in the left bottom corner of the plot. The coexistence region (shaded in gray) contains P and G compositions consistent with both amyloidogenic and elastomeric properties. Elastomeric proteins, including the domains of elastin, major ampullate spindroin (MaSp) 2, flagelliform silk, the elastic domains of mussel byssus thread, and abductin, appear above a composition threshold (upper dashed line). Amyloidogenic sequences are primarily found below the PG-threshold, along with rigid lizard egg shells, tubulliform silk (TuSp1), a protective silk for spider eggs, and aciniform silk (AcSp), used for wrapping prey. The coexistence region contains amyloid-like peptides as well as the elastomeric adhesive produced by the frog Notaden bennetti, the PEVK domains of titin, wheat glutenin protein, and the strongest spider silks, namely MaSp1 and minor ampullate spindroin (MiSp). Figure reproduced from ref. 130 Abbreviations: AcSp, aciniform silk; MaSp, major ampullate spindroin; MiSp, minor ampullate spindroin; TuSp1, tubulliform silk.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.