Table 1. Model acceptor glycosylations.
| Acceptor | N a | F b |
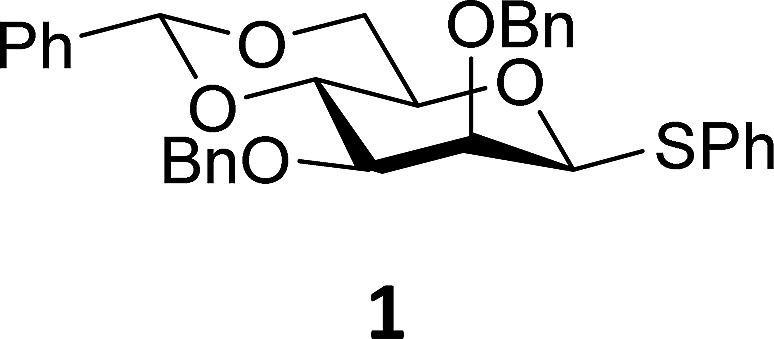
|
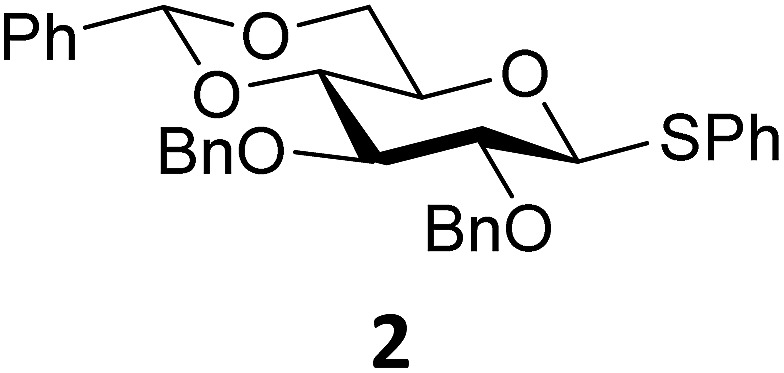
|
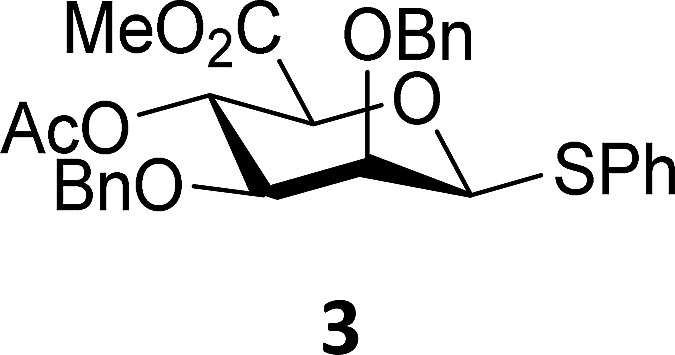
|
| Product, α : β (yield) c | Product, α : β (yield) c | Product, α : β (yield) c | |||
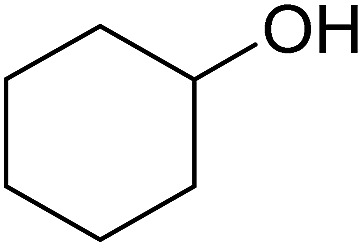
|
— | — | 1A | 2A | 3A |
| 1 : 6 | 1 : 5 | 1 : 8 | |||
| (96%) | (71%) | (83%) | |||
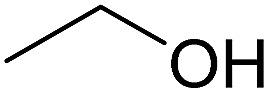
|
7.44 | 0.01 | 1B | 2B | 3B |
| 1 : 5 | 1 : 10 | 1 : 8 | |||
| (70%) | (68%) | (95%) | |||
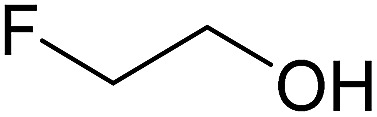
|
— | 0.15 | 1C | 2C | 3C |
| 1 : 5 | 1 : 3 | 1 : 6 | |||
| (86%) | (70%) | (70%) | |||
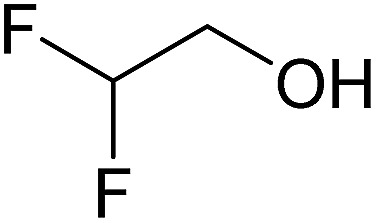
|
— | 0.29 | 1D | 2D | 3D |
| 1 : 5 | 5 : 1 | 1 : 5 | |||
| (90%) | (70%) | (87%) | |||
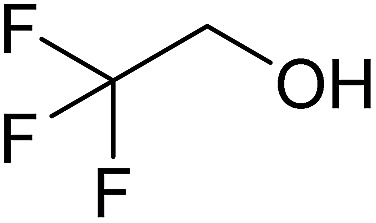
|
1.11 | 0.38 | 1E | 2E | 3E |
| 1 : 4 | >20 : 1 | 1 : 2.5 | |||
| (78%) | (64%) | (85%) | |||
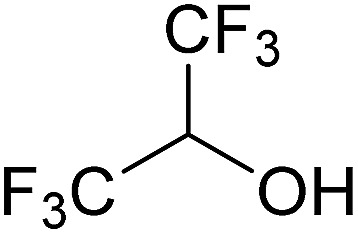
|
–1.93 | — | 1F | 2F | 3F |
| 3 : 1 | >20 : 1 | 1 : 1 | |||
| (56%) | (65%) | (52%) | |||
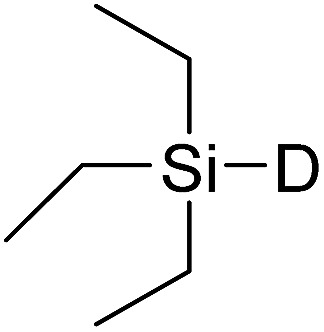
|
3.58 | — | 1G | 2G | 3G |
| <1 : 20 | >20 : 1 | <1 : 20 | |||
| (60%) | (79%) | (95%) | |||
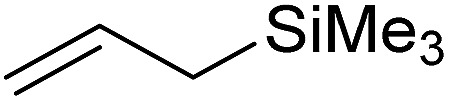
|
1.68 | — | 1H | 2H | 3H |
| <1 : 20 | >20 : 1 | <1 : 20 | |||
| (44%) d | (42%) d | (40%) d |
