Abstract
Background
Pain is a significant public health concern, and current pharmacological treatments have problematic side effects and limited effectiveness. N-methyl-D-aspartate (NMDA) glutamate receptor antagonists have emerged as one class of candidate treatments for pain because of the significant contribution of glutamate signalling in nociceptive processing.
Methods
This study compared effects of the NMDA receptor antagonists ketamine and MK-801 in assays of pain-stimulated and pain-depressed behaviour in rats. The nonsteroidal anti-inflammatory drug ketoprofen was examined for comparison as a positive control. Intraperitoneal injection of dilute acid served as an acute visceral noxious stimulus to stimulate a stretching response or depress intracranial self-stimulation (ICSS) in male Sprague–Dawley rats.
Results
Ketamine (1.0–10.0 mg/kg) blocked acid-stimulated stretching but failed to block acid-induced depression of ICSS, whereas MK-801 (0.01–0.1 mg/kg) blocked both acid-stimulated stretching and acid-induced depression of ICSS. These doses of ketamine and MK-801 did not alter control ICSS in the absence of the noxious stimulus; however, higher doses of ketamine (10 mg/kg) and MK-801 (0.32 mg/kg) depressed all behaviour. Ketoprofen (1.0 mg/kg) blocked both acid-induced stimulation of stretching and depression of ICSS without altering control ICSS.
Conclusion
These results support further consideration of NMDA receptor antagonists as analgesics; however, some NMDA receptor antagonists are more efficacious at attenuating pain-depressed behaviours.
What does this study add?
NMDA receptor antagonists produce dissociable effects on pain-depressed behaviour.
Provides evidence that pain-depressed behaviours should be considered and evaluated when determining the antinociceptive effects of NMDA receptor antagonists.
1. Introduction
N-methyl-D-aspartate (NMDA) glutamate receptors play a key role in transmission of nociceptive information in the central nervous system (Bleakman et al., 2006;Wozniak et al., 2012), and NMDA receptor antagonists are under consideration as candidate analgesics (Niesters and Dahan, 2012). In support of this consideration, systemic administration of ketamine or the more selective NMDA antagonist MK-801 produced antinociception in several preclinical animal models (Millan and Seguin, 1994; Fundytus, 2001; Sabetkasaie et al., 2007). However, the analgesic efficacy of NMDA antagonists in clinical research has been mixed and depends on variables that include (1) route of administration; (2) treatment duration; (3) pain state; and (4) pain measurement (Niesters et al., 2014; Sawynok, 2014). Moreover, the clinical utility of NMDA receptor antagonists is constrained by concerns regarding side effects including psychotomimesis and abuse liability.
Preclinical studies may help to clarify determinants of NMDA antagonist analgesic effectiveness, and one important variable is the behavioural manifestation of pain under investigation. Virtually all preclinical studies with NMDA antagonists have employed assays of pain-stimulated behaviours, which can be defined as behaviours that increase in frequency, rate or intensity after delivery of a noxious stimulus (Negus et al., 2010a; Negus, 2013). In rodents, for example, ketamine and MK-801 decreased stretching behaviour stimulated by intraperitoneal acid administration and flinching behaviours stimulated by formalin injection into the hindpaw (Takahashi et al., 1987; Finck et al., 1988; Millan and Seguin, 1994; Bulutcu et al., 2002; Sawynok and Reid, 2002; Malec and Poleszak, 2005;Sabetkasaie et al., 2007). However, exclusive reliance on assays of pain-stimulated behaviour in preclinical drug evaluation can be problematic for several reasons. For example, pain-stimulated behaviours can be reduced not only by reductions in sensitivity to the noxious stimulus but also by motor impairment (Negus et al., 2010a). Novel assays of pain-depressed behaviours have emerged to complement more conventional procedures (Negus et al., 2010a; Negus, 2013). Pain-depressed behaviours are defined as behaviours that decrease in frequency, rate or intensity after delivery of a noxious stimulus. Assays that measure pain-depressed behaviours can be useful in part because pain states often produce clinically relevant impairment of behaviours like feeding, locomotion or positively reinforced operant behaviour (Cleeland and Ryan, 1994; Turk et al., 2008).
One recent study reported that ketamine restored pain-related depression of swimming and sucrose preference induced in rats by spared nerve injury (Wang et al., 2011); however, research on NMDA antagonist effects in assays of pain-depressed behaviour is just beginning. This study compared effects of ketamine and MK-801 in rats using complementary assays of intraperitoneal acid-stimulated stretching and acid-depressed intracranial self-stimulation (ICSS) that have been used to evaluate antinociceptive effects of other drugs including opioids (Pereira Do Carmo et al., 2009; Negus et al., 2012a, b; Leitl et al., 2014;Altarifi et al., 2015; Miller et al., 2015a, b), cannabinoids (Kwilasz and Negus, 2012; Kwilasz et al., 2014) and monoamine reuptake inhibitors (Rosenberg et al., 2013; Miller et al., 2015a, b). We hypothesized that ketamine and MK-801 would block both acid-stimulated stretching and acid-induced depression of ICSS.
2. Methods
2.1 Subjects
A total of 26 adult male Sprague–Dawley rats (Harlan Laboratories Inc, Frederick, MD, USA) were used in studies of lactic acid-stimulated stretching (n = 16) and lactic acid-depressed ICSS (n = 10). Rats weighed between 300 and 360 g at the time of surgery, and they were individually housed in plastic cages in the vivarium with a 12-h light/dark cycle with lights on 6:00 a.m. All rats had free access to food and water except during experimental sessions. Procedures complied with the Guide for the Care and Use of Laboratory Animals and were approved by the Virginia Commonwealth University Institutional Animal Care and Use Committee.
At the beginning of the study, all rats were implanted with a stainless-steel electrode under isoflurane anaesthesia as described previously (Rosenberg et al., 2013; Hillhouse et al., 2014). The cathode of each electrode (Plastics One, Roanoke, VA, USA) was 0.25 mm in diameter and covered with polyamide insulation, except at the flattened tip. The anode was 0.124 mm in diameter and uninsulated. The cathode was implanted in the left medial forebrain bundle at the level of the lateral hypothalamus (2.8 mm posterior and 1.7 mm lateral from the bregma, and 8.8 mm below the skull). The anode was wrapped around one of three skull screws to serve as the ground, and the skull screws and electrode assembly were secured with orthodontic resin. Rats received ketoprofen (5 mg/kg i.p. for 2 days) as a postoperative analgesic and were allowed to recover for at least 7 days before commencing ICSS training.
2.2 Assay of lactic acid-depressed ICSS
2.2.1 Apparatus
Intracranial self-stimulation studies were conducted in 12 sound-attenuating chambers that contained operant conditioning chambers (29.2 × 30.5 × 24.1 cm) equipped with a response lever (4.5-cm wide, extended 2.0 cm through the centre of one wall, 3 cm off the floor), stimulus lights (three lights coloured red, yellow and green positioned 7.6 cm directly above the lever), a house light and an ICSS stimulator (Med-Associates, St Albans, VT, USA). Electrodes were connected to the stimulator through bipolar cables and a commutator (Model SL2C; Plastics One). Programming of behavioural sessions and data collection were computer controlled by Med-State software (Med PC, Version 4.1; Med-Associates).
2.2.2 Behavioural procedure
All rats were initially exposed to ICSS training using procedures similar to those described previously to establish lever press responding for pulses of electrical brain stimulation (0.5-s train of 0.1 ms square-wave cathodal pulses) under a fixed-ratio 1 (FR 1) schedule (Negus and Miller, 2014). During initial training, the frequency of stimulation was held constant at 158 Hz, and the intensity was adjusted individually in each rat to the lowest intensity sufficient to maintain an ICSS rate >30 stimulations per minute. Frequency manipulations were then introduced, and the terminal schedule consisted of sequential 10-min components. During each component, a descending series of 10 brain stimulation frequencies was presented, with a 60-s trial at each of 10 frequencies (158 to 56 Hz in 0.05-log increments). Each frequency trial began with a 10-s timeout, during which the house light was off and responding had no scheduled consequences. During the last 5 s of this timeout, five noncontingent stimulations were delivered once per second at the frequency available during that trial, and the lever lights were illuminated during each stimulation. This noncontingent stimulation was then followed by a 50-s ‘response’ period, during which the house light was illuminated, and each lever press produced electrical stimulation and illumination for 0.5 s of the coloured stimulus lights over the lever. Training continued with presentation of three to six sequential components per day, and stimulation intensities were again adjusted individually for each rat, until rats reliably responded at rates ≥50% maximum control rates (MCRs; see Data analysis) for at least three and no more than six trials of all components for at least three consecutive days. Stimulation intensities were then held constant for the remainder of the study in each rat (range: 100–280 μA). In general, rats were implanted with electrodes and exposed to ICSS in groups of 12–16, and the first six rats in each group to meet training criteria advanced to ICSS pharmacological testing. The remaining rats that failed to meet training criteria were assigned to assays of acid-stimulated stretching (see below). Overall, 10 rats completed ICSS studies and 16 rats completed stretching studies. Additionally, rats were habituated to saline injections until these injections had no significant effect on ICSS frequency-rate curves as determined by two-way analysis of variance (ANOVA; see Data Analysis).
Testing was conducted using a within-subject experimental design that has been used previously to evaluate antinociceptive effects of other drugs including opioids (Pereira Do Carmo et al., 2009; Negus et al., 2012a, b; Leitl et al., 2014; Altarifi et al., 2015; Miller et al., 2015a, b), cannabinoids (Kwilasz and Negus, 2012; Kwilasz et al., 2014) and monoamine reuptake inhibitors (Rosenberg et al., 2013; Miller et al., 2015a, b). ICSS test sessions for dose–effect testing consisted of six sequential components. The first component of each test session was considered an acclimation component, and data from this component were discarded. Data from the second and third ‘baseline’ components were used to calculate control parameters of frequency-rate curves for that test session in that rat (see Data Analysis). Immediately after completion of the baseline components, rats were taken out of the ICSS chambers, administered drug or vehicle (i.p.) and placed back into their home cages. After the designated pretreatment time elapsed, 1.8% lactic acid or its vehicle was administered i.p. in a volume of 1 mL/kg, and rats were immediately placed back into the ICSS chambers for three test components (10 min each, 30 min total). This testing period was chosen to match the session length for stretching studies (see below). Doses and pretreatment times (ketamine: 1.0–10.0 mg/kg, 10 min; MK-801: 0.01–0.32 mg/kg, 15 min; ketoprofen: 1.0 mg/kg, 30 min) were based on empirical results and on previous studies (Negus et al., 2012a; Hillhouse et al., 2014), and dose order across rats was counterbalanced using a Latin-square design. Each drug was tested in a group of five rats. If ICSS performance remained stable in a given rat after completion of testing with the initial drug, then the rat was advanced to testing with the next drug. There was at least a 7-day washout period between drugs. For ketamine, all rats were drug naïve. For MK-801, all rats had been tested previously with ketamine. For ketoprofen, four rats were drug naïve, and one rat had been tested previously with ketamine and MK-801. Testing was conducted twice per week (typically Tuesday and Friday) with at least 72 h between drug injections. Additionally, 1.8% lactic acid injections separated by 1 week to match acid-stimulated stretching studies.
2.2.3 Data analysis
Data were analysed using procedures described previously (Rosenberg et al., 2013; Negus and Miller, 2014). The primary dependent variable was the reinforcement rate in stimulations/trial during each frequency trial. To normalize these raw data, reinforcement rates from each trial were converted into the per cent MCR, with MCR defined as the mean of the maximal number of stimulations/trial observed in any frequency trial during the second and third baseline components for each test day in each rat. Thus, % MCR for each trial was calculated as (stimulations during a frequency trial/MCR)*100. Normalized data from the frequency trials of consecutive test components were then averaged across rats for display and for statistical analysis using two-way repeated measures ANOVA, with drug dose as one factor and ICSS frequency as the other factor. A significant ANOVA was followed by a Holm–Sidak post hoc test, and the criterion for significance was set at p < 0.05.
To provide an additional summary of ICSS performance, the total number of stimulations delivered across all 10 frequency trials was determined for each component. The average number of total stimulations per test component was expressed as a percentage of the average number of total stimulations per component during the second and third baseline components using the equation % Baseline Total Stimulations = (average test stimulations per component/average baseline stimulations per component) *100. These values were then averaged across rats in each experimental condition for visual display. Additionally, these data were also used to quantify blockade of acid-induced depression of ICSS. Specifically, ‘per cent acid blockade’ was quantified using the equation ([test-acid]/[baseline-acid])*100, where ‘test’ was the total number of ICSS stimulations per component after treatment with drug + acid, ‘acid’ was the total number of stimulations per component after treatment with vehicle + acid, and ‘baseline’ was the total number of stimulations per component during the second and third baseline components (daily baseline). For all drugs producing dose-dependent effects that exceeded 50% acid blockade, a linear regression in GraphPad Prism 6.0 (La Jolla, CA, USA) was used to calculate an ED50 and 95% confidence intervals (CI), with ED50 defined as the effective dose producing 50% acid blockade. ED50 values were considered to be significantly different if 95% CIs did not overlap. A value of 100% acid blockade indicated complete blockade of acid-induced depression of ICSS. Values greater than 100% acid blockade indicated facilitation of ICSS above baseline levels, and values below 0% indicated exacerbation of acid-induced depression of ICSS.
2.3 Assay of lactic acid-stimulated stretching
2.3.1 Behavioural procedure
To complement studies in the assay of acid-depressed ICSS, 16 rats that failed to meet ICSS training criteria within 4 weeks were used in studies of lactic acid-stimulated stretching as described previously (Pereira Do Carmo et al., 2009; Negus et al., 2012a, b; Rosenberg et al., 2013). During test sessions, each rat received an i.p. injection of the test drug followed first by a designated pretreatment interval and then by i.p. injection of 1.8% lactic acid in a volume of 1 mL/kg. Immediately after the second injection, rats were placed into an acrylic test chamber (31.0 × 20.1 × 20.0 cm) for a 30-min observation period, and the number of stretches was counted. A stretch was operationally defined as a contraction of the abdomen followed by extension of the hindlimbs. Drugs, doses and pretreatment times were identical to those used in studies of acid-depressed ICSS, and dose order across rats was counterbalanced using a Latin-square design. Each drug was tested in a group of seven rats. For ketamine and MK-801, all rats were drug naive. For ketoprofen, two rats were drug naïve and five rats had been tested previously with MK-801. Test sessions were conducted once per week.
2.3.2 Data analysis
The primary dependent variable was the number of stretches counted during each observation period in each rat. Raw data were normalized to the vehicle control in each rat using the equation Per cent Control Stretching = (drug/vehicle)* 100, where ‘drug’ was the number of stretches observed after a test drug dose + acid, and ‘vehicle’ was the number of stretches after vehicle + acid. Data for ketamine and MK-801 were then analysed using a one-way repeated measures ANOVA with drug dose as the within-subjects factor, and Holm–Sidak post hoc tests were conducted after all significant ANOVAs. A paired t-test was used for ketoprofen. The criterion for significance was set to p < 0.05 for all statistical tests. To calculate the ED50 values and 95% CIs for ketamine and MK-801, dose–effect data were analysed by linear regression in GraphPad Prism 6.0, and the ED50 was defined as the effective dose producing 50% control stretching.
2.4 Drugs
The noncompetitive NMDA receptor antagonists (±) ketamine HCl and (+) MK-801 hydrogen maleate (both from Sigma Aldrich, St. Louis, MO, USA) were dissolved in 0.9% physiological saline. The nonsteroidal anti-inflammatory drug ketoprofen propionate (Spectrum Chemical, Gardena, CA, USA) was dissolved in bacteriostatic water. Lactic acid (Sigma Aldrich) was diluted in bacteriostatic water. All drugs were administered intraperitoneally at a volume of 1.0 mL/kg.
3. Results
3.1 Effects of noncompetitive NMDA receptor antagonists on acid-stimulated stretching
Administration of the noxious stimulus (i.p. injection of 1.0 mL/kg 1.8% lactic acid) produced a mean ± SEM of 15.44 ± 0.88 stretches across all 16 rats. The mean ± SEM number of stretches for each drug vehicle + 1.8% lactic acid were as follows: ketamine (n = 7), 14.71 ± 0.88; MK-801 (n = 7), 16.21 ± 2.09; ketoprofen (n = 7), 15.93 ± 1.74. Fig 1 shows the effects of ketamine, MK-801 and ketoprofen on per cent control stretching. Ketamine (1–10 mg/kg) and MK-801 (0.01–0.32 mg/kg) produced dose-dependent decreases in acid-stimulated stretching, and ED50 values are shown in Table 1. The 1.0 mg/kg dose of ketoprofen also significantly decreased acid-stimulated stretching.
Figure 1.
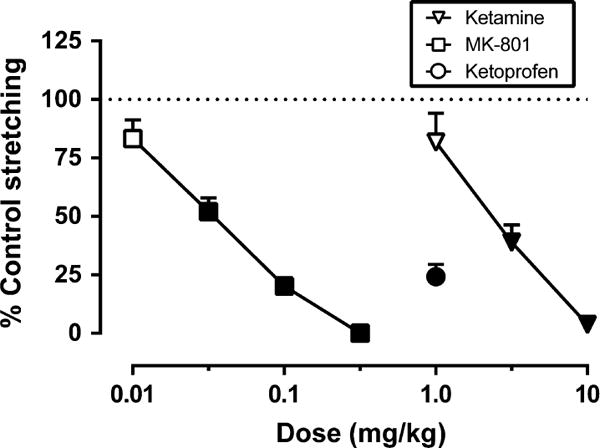
Effects of ketamine, MK-801 and ketoprofen on acid-stimulated stretching. Abscissa: Drug dose in mg/kg (log scale). Ordinate: Per cent control stretching observed after vehicle administration. All points show mean ± SEM for seven rats, and ED50 values are reported in Table 1. There was a significant main effect of dose for ketamine (F[3,18] = 34.52, p < 0.001), MK-801 (F[4,24] = 25.12, p < 0.001) and ketoprofen (t[6] = 7.34, p < 0.001). Filled points indicate significantly different from vehicle control.
Table 1.
ED50 values in mg/kg (95% confidence intervals) for the NMDA antagonists ketamine and MK-801 in the assays of acid-stimulated stretching or acid-depressed ICSS.
| Drug | Acid-stimulated stretching | Acid-depressed ICSS |
|---|---|---|
| Ketamine | 2.47 (1.84–3.32) | Inactive |
| MK-801 | 0.036 (0.029–0.045) | 0.013 (0.003–0.049) |
A single dose of ketoprofen (1.0 mg/kg), therefore an ED50 value was not calculated for ketoprofen. Inactive indicates a failure to produce at least 50% acid blockade in acid-depressed ICSS.
3.2 Effects of noncompetitive NMDA receptor antagonists on acid-depressed ICSS
Prior to each test session in each rat, a baseline frequency-rate curve was determined to establish the MCR and total number of stimulations per component for that session. Across all 10 rats used in ICSS studies, the mean ± SEM MCR was 60.16 ± 2.69 stimulations per trial, and the mean ± SEM total number of stimulations per component was 261.02 ±16.75. Fig 2 shows that the acid noxious stimulus depressed ICSS. Under control conditions (vehicle + vehicle), electrical brain stimulation maintained a frequency-dependent increase in responding, and treatment with i.p. lactic acid produced a rightward and downward shift in the ICSS frequency-rate curve and a decrease in total stimulations per component. Specifically, treatment with lactic acid decreased ICSS rates across the six highest frequencies (89–158 Hz) and decreased the number of total stimulations per component to 59% of baseline.
Figure 2.
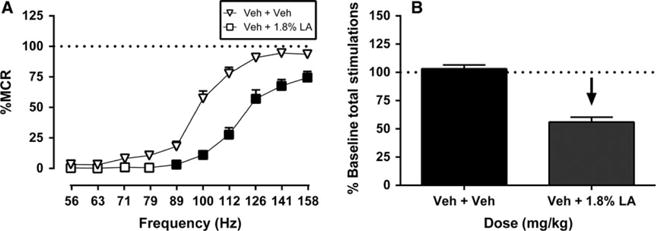
Acid-induced depression of ICSS. Left panel (A) shows the effects of pretreatment with vehicle + vehicle (Veh + Veh) and vehicle + 1.8% lactic acid (Veh + 1.8% LA) on full ICSS frequency-rate curves for all 10 rats used in ICSS studies. Abscissa: Frequency of electrical brain stimulation in Hz (log scale). Ordinate: Per cent maximum control rate (%MCR). Filled points represent frequencies at which ICSS rates after Veh + 1.8% LA were significantly lower than rates after Veh + Veh as determined by a two-way ANOVA followed by a Holm–Sidak post hoc test, p < 0.05. Right panel (B) shows summary ICSS data for lactic acid effects on the total number of stimulations per component delivered across all frequencies. Abscissa: Treatment condition. Ordinate: Per cent baseline stimulations per test component. The downward arrow indicates a significant acid-induced decrease in ICSS relative to Veh + Veh for at least one brain stimulation frequency as determined by analysis of full frequency-rate curves in the left panel. All data show mean ± SEM for 10 rats. Statistical results for the left panel (A) are as follows: Significant main effect of frequency (F[9,126] = 226.70, p < 0.001) and treatment (F[1,14] = 184.60, p < 0.001) and a significant interaction (F[9,126] = 14.41, p < 0.001).
Fig 3 shows the effects ketamine (n = 5) and MK-801 (n = 5) on ICSS in the absence and presence of the noxious stimulus. In the absence of the noxious stimulus, ketamine depressed ICSS. Treatment with 1.0 and 3.2 mg/kg ketamine had no significant effect, but 10.0 mg/kg ketamine decreased ICSS rates across a board range of frequencies (100–141 Hz) (Fig 3A). When ketamine was administered as a pre-treatment to lactic acid, ketamine did not significantly alter ICSS and failed to block acid-induced depression of ICSS (Fig 3B). MK-801 also depressed ICSS in the absence of the noxious stimulus. Treatment with 0.01–0.1 mg/kg MK-801 did not significantly alter ICSS, but 0.32 mg/kg MK-801 depressed ICSS across the five highest frequencies (100–158 Hz) (Fig 3D). When MK-801 was administered as a pretreatment to lactic acid, MK-801 produced both increasing and decreasing effects on ICSS (Fig 3E). Specifically, 0.1 mg/kg MK-801 increased rates of ICSS at intermediate frequencies (100–112 Hz) to attenuate acid-induced depression of ICSS. Conversely, 0.32 mg/kg MK-801 exacerbated acid-induced depression of ICSS at the three highest frequencies (126–158 Hz).
Figure 3.
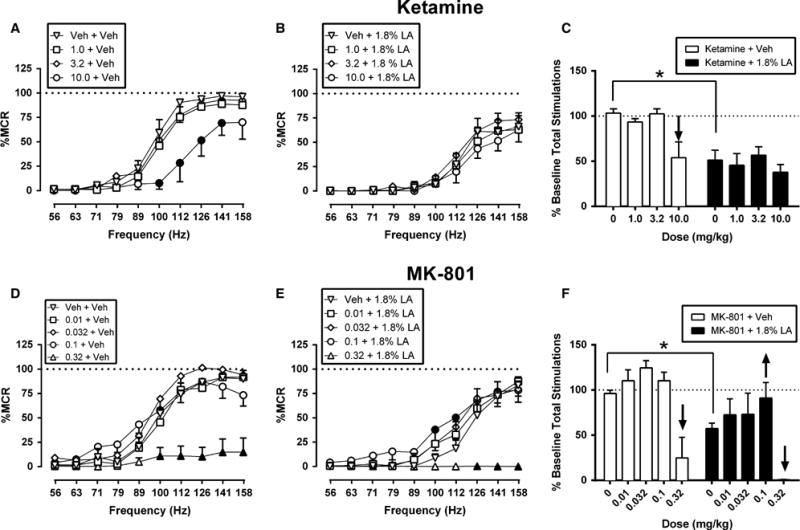
Effects of the ketamine (A–C) and MK-801 (D–F) on control and acid-depressed ICSS. Left and centre panels show drug effects on full ICSS frequency-rate curves when drugs were administered as a pretreatment to acid vehicle (left panels A, D) or 1.8% lactic acid (centre panels B, E). Abscissae: Frequency of electrical brain stimulation in Hz (log scale). Ordinates: Per cent maximum control rate (%MCR). Filled points represent frequencies at which ICSS rates after test drug treatment were significantly different from rates after treatment with Veh + Veh (A, D) or Veh + 1.8% LA (B, E) as determined by a two-way ANOVA followed by a Holm–Sidak post hoc test, p < 0.05. Right panels (C, F) show summary ICSS data for drug effects on the total number of stimulations delivered across all frequencies per test component when drugs were administered as a pre-treatment to acid vehicle (open bars) or 1.8% lactic acid (filled bars). Abscissae: Drug dose (mg/kg). Ordinates: Per cent baseline stimulations per test component. Upward/downward arrows indicate that the drug dose produced a significant increase/decrease in ICSS for at least one brain stimulation frequency as determined by analysis of full frequency-rate curves in the left and centre panels. All data show mean ± SEM for five rats. Statistical results for the left and centre panels are as follows: (A) ketamine + vehicle: Significant main effect of frequency (F[9,36] = 120.50, p < 0.001) and treatment (F[3,12] = 10.22, p = 0.001) and a significant interaction (F[27,108] = 2.88, p < 0.001). (B) ketamine +1.8% LA: Significant main effect of frequency (F[9,36] = 42.15,p < 0.001) but not dose (F[3,12] = 0.50, p = 0.69); the interaction was not significant (F[27,108] = 0.39, p = 0.99). (D) MK-801 + vehicle: Significant main effect of frequency (F[9,36] = 117.00, p < 0.001) and treatment (F[4,16] = 19.41, p < 0.001) and a significant interaction (F[36,144] = 8.13, p < 0.001). (E) MK-801 +1.8% LA: Significant main effect of frequency (F[9,36] = 57.40, p < 0.001) and dose (F[4,16] = 10.05, p < 0.001) and a significant interaction (F[36,144] = 8.40, p < 0.001). *Indicates significantly different from Veh + Veh in the right panels as determined by a paired t-test (p < 0.05).
Fig 4 shows the effects ketoprofen (n = 5) on ICSS in the absence and presence of the lactic acid noxious stimulus. Ketoprofen (1.0 mg/kg) did not alter ICSS in the absence of lactic acid (Fig 4A). When administered as a pretreatment to lactic acid, 1.0 mg/kg ketoprofen ameliorated acid-induced depression of ICSS (Fig 4B) and increased ICSS rates across a range of intermediate and high frequencies (89–141 Hz).
Figure 4.
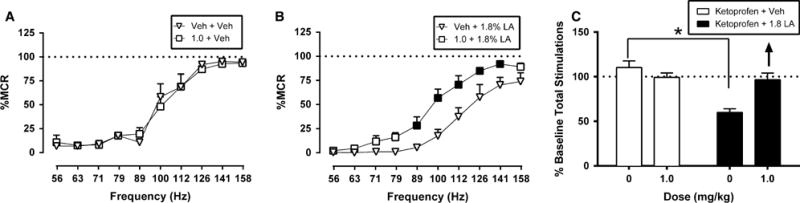
Effects of ketoprofen on control and acid-depressed ICSS. Left and centre panels show drug effects on full ICSS frequency-rate curves when ketoprofen or its vehicle was administered as a pretreatment to acid vehicle (left panel A) or 1.8% lactic acid (centre panel B). Filled points represent frequencies at which ICSS rates after ketoprofen treatment were significantly different from rates after Veh + Veh (A) or Veh + 1.8% LA (B) as determined by a two-way ANOVA followed by a Holm–Sidak post hoc test, p < 0.05. Right panel (C) shows summary ICSS data for drug effects on the total number stimulations delivered across all frequencies per test component when ketoprofen was administered as a pretreatment to acid vehicle (open bars) or 1.8% lactic acid (filled bars). Other details as in Fig. 3. All data show mean ± SEM for five rats. Statistical results for the left and centre panels are as follows: (A) ketoprofen + vehicle: Significant main effect of frequency (F[9,36] = 44.79, p < 0.001) but not treatment (F[1,4] = 0.12, p = 0.74); the interaction was not significant (F[9,36] = 0.79, p = 0.63). (B) ketoprofen + 1.8% LA: Significant main effect of frequency (F[9,36] = 45.63, p < 0.001) and treatment (F[1,4] = 60.63, p = 0.001) and a significant interaction (F[9,36] = 2.82, p < 0.05). *Indicates significantly different from Veh + Veh in the right panels as determined by a paired t-test (p < 0.05).
Fig 5 shows the effects of ketamine, MK-801 and ketoprofen expressed as ‘per cent acid blockade’ on acid-depressed ICSS. Ketamine failed to attenuate acid-induced depression of ICSS, and only one dose of ketoprofen (1.0 mg/kg) was tested; therefore, ED50 values could not be calculated for these drugs. MK-801 produced a significant blockade of acid-depressed ICSS at the 0.1 mg/kg dose, and the ED50 value is shown in Table 1. MK-801 displayed similar potencies to block acid-induced depression of ICSS and acid-induced stimulation of stretching as indicated by overlapping confidence limits for ED50 values.
Figure 5.
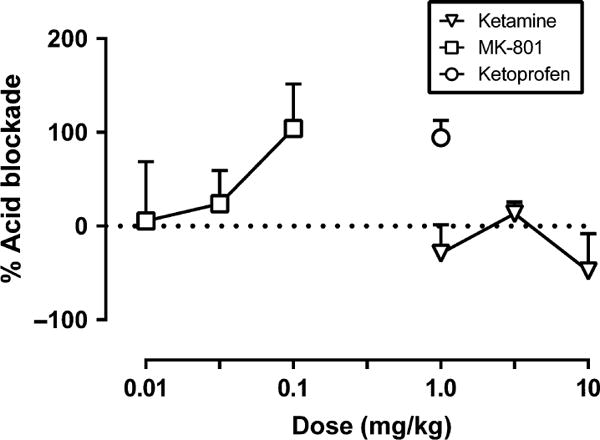
Effects of ketamine, MK-801 and ketoprofen on acid-depressed ICSS expressed as % acid blockade. Abscissa: Drug dose (mg/kg). Ordinate: Per cent blockade of acid-induced depression of ICSS. All data show mean ± SEM for five, and ED50 values are reported in Table 1.
4. Discussion
The present study compared the antinociceptive effects of the noncompetitive NMDA receptor antagonists ketamine and MK-801 in assays of acid-stimulated stretching and acid-depressed ICSS. There were three main findings. First, in agreement with previous studies, both ketamine and MK-801 produced antinociceptive effects in an assay of pain-stimulated behaviour, in which ketamine and MK-801 dose-dependently decreased the number of acid-induced stretches. Second, MK-801 but not ketamine, produced antinociceptive effects in the assay of pain-depressed behaviour; however, the effective dose range for MK-801 was narrow. Specifically, only the 0.1 mg/kg dose of MK-801 attenuated acid-depressed ICSS, whereas ketamine failed to alter acid-depressed ICSS at any dose tested. Furthermore, the high dose of either ketamine or MK-801 depressed all behaviours. Finally, the NSAID ketoprofen attenuated both acid-stimulated stretching and acid-depressed ICSS. These results suggest that MK-801 may be more effective than ketamine to attenuate both pain-depressed and pain-stimulated behaviours.
4.1 Ketoprofen effects on acid-stimulated stretching and acid-depressed ICSS
The effectiveness of ketoprofen to block both acid-stimulated stretching and acid-induced depression of ICSS agrees with previous studies with ketoprofen in these two procedures (Kwilasz and Negus, 2012; Negus et al., 2012a; Leitl et al., 2014) and with the clinical effectiveness of ketoprofen as a clinically effective analgesic drug under conditions of inflammatory pain (Veys, 1991; Caldwell, 1994; Kokki et al., 1999). Moreover, a similar profile of drug effects on acid-induced stimulation of stretching and depression of ICSS has been produced by morphine and other mu opioid analgesics (Pereira Do Carmo et al., 2009; Negus et al., 2010b; Leitl et al., 2014; Altarifi et al., 2015). These results with ketoprofen as a positive control illustrate the profile of effects produced by a clinically effective analgesic drug, and effects of NMDA antagonists were compared to effects of ketoprofen.
4.2 NMDA receptor antagonist effects on acid-stimulated stretching
Like ketoprofen, both ketamine and MK-801 dose-dependently decreased acid-stimulated stretching in rats. These results are consistent with previous reports that both noncompetitive and competitive NMDA receptor antagonists produce antinociceptive effects in assays of pain-stimulated behaviours. For example, the noncompetitive NMDA receptor antagonists ketamine (Ryder et al., 1978; Takahashi et al., 1987; Finck et al., 1988; Bulutcu et al., 2002; Jahangiri et al., 2013), MK-801 (Millan and Seguin, 1994; Seguin et al., 1995; Malec and Poleszak, 2005; Suardiaz et al., 2007) and memantine (Millan and Seguin, 1994), and the competitive NMDA receptor antagonists CGS 19755 and CPP (3-((+)-2-carboxypi-perazin-4-yl)-propyl-l-phosphonic acid) (Millan and Seguin, 1994) attenuate acid-stimulated stretching in mice. Moreover, these same NMDA antagonists have also been shown to block formalin-induced stimulation of paw licking and flinching in mice (Millan and Seguin, 1994; Seguin et al., 1995; Bulutcu et al., 2002; Sabetkasaie et al., 2007). In rodent models of neuropathic pain, NMDA receptor antagonist also decrease hypersensitive paw withdrawal responses induced by application of thermal and mechanical stimuli (Christoph et al., 2006; Swartjes et al., 2011; Andreasen et al., 2013). Reductions in these pain-stimulated behaviours are often interpreted as antinociceptive effects; however, drug-induced decreases in pain-stimulated behaviours may reflect impaired ability to emit the motor response rather than reduced sensitivity to the noxious stimulus. Consistent with this possibility, the doses of MK-801 and ketamine that produced maximal decreases in stretching (0.32 and 10 mg/kg, respectively) in the present study also produced significant decreases in ICSS. Thus, in the present study, we evaluated the effects of ketamine and MK-801 on pain-depressed behaviours to provide additional insight into the antinociceptive profile of these NMDA receptor antagonists.
4.3 NMDA receptor antagonist effects on acid-induced depression of ICSS
Similar to ketoprofen, MK-801 block acid-induced depression of ICSS as well as acid-stimulated stretching suggesting that MK-801 did produce an antinociceptive decrease in sensitivity to the acid noxious stimulus. This finding supports further consideration of NMDA receptor antagonists as candidate analgesics. However, it should be noted that MK-801 had a narrow therapeutic window, and only 0.1 mg/ kg significantly attenuated both acid-induced stimulation of stretching and depression of ICSS. A higher dose of 0.32 mg/kg produced a greater reduction in acid-stimulated stretching but also depressed ICSS in the absence of the noxious stimulus and exacerbated acid-induced depression of ICSS, suggesting that this high MK-801 dose produced general motor impairment.
In contrast to the effects of ketoprofen and MK-801, ketamine failed to exert a significant effect on acid-depressed ICSS. The inability of ketamine to attenuate acid-induced depression of ICSS in the present study contrasts the finding that ketamine attenuated pain-related depression of sucrose preference and swimming behaviour in a spared nerve injury model of neuropathic pain in rats (Wang et al., 2011). A major difference between these studies is the type of pain state used to produce a depression of behaviours. Specifically, in the present study, we used an acute visceral noxious stimulus to depress ICSS, whereas Wang et al. (2011) used a neuropathic manipulation (i.e. spared nerve injury) to depress sucrose preference and swimming behaviour. Consequently, these results suggest that ketamine may be more effective to alleviate manifestations of sustained neuropathic pain than acute pain. Similar results have been found in clinical research such that ketamine may be more effective for neuropathic pain (Backonja et al., 1994; Felsby et al., 1996; Leung et al., 2001; Jørum et al., 2003), spinal cord injuries (Amr, 2010; Kim et al., 2013) and complex regional pain syndrome (Schwartzman et al., 2009; Sigtermans et al., 2009) as compared to visceral pain states. For example, several small sample (n = 6−12) double-blind clinical studies found that a single intravenous infusion of ketamine can reduce allodynia, hyperalgesia and spontaneous/ongoing pain in patients suffering from neuropathic pain (Backonja et al., 1994;Felsby et al., 1996; Leung et al., 2001; Jørum et al., 2003). On the other hand, oral administration of the more potent isomer S-ketamine (25 or 50 mg) did not alter pain scores in a gastric-distension model of acute visceral pain (Kuiken et al., 2004). Similar to ketamine, selective serotonin or norepinephrine reuptake inhibitors (Rosenberg et al., 2013), cannabinoid 1 receptor agonists (Kwilasz and Negus, 2012), endocannabinoid enzyme inhibitors (Kwilasz et al., 2014) and kappa opioid agonists (Negus et al., 2010b, 2012a) all fail to block aciddepressed ICSS, but are successful at blocking acid-stimulated stretching.
The present results in the assay of acid-depressed ICSS suggest a dissociation in effects of MK-801 and ketamine despite the general categorization of both drugs as NMDA receptor antagonists. Moreover, a similar dissociation of behavioural effects of MK-801 and ketamine has been shown in rats in assays of conditioned and unconditioned behaviours (McMillan et al., 1992;Gilmour et al., 2009;Smith et al., 2011; Hillhouse and Porter, 2014). The pharmacological differences in affinity and selectivity of MK-801 and ketamine for NMDA receptors may account for the behavioural difference found in the present and previous studies. For example, MK-801 binds to NMDA receptors with high affinity (Ki = 2.5 nM) and selectively (Bresink et al., 1995; Nishimura et al., 1998a), whereas ketamine has low affinity (Ki = 1.190 nM) for NMDA receptors and appreciable affinity for several G-protein-coupled receptors (GPCRs). For example, ketamine binds to mu (Ki = 26.8 μM), kappa (Ki = 85.2 μM) and delta (Ki = 101.0 μM) opioid receptors as well as muscarinic M1 (Ki = Ki = 45.0 μM) and sigma receptors (Ki = 66.0 μM) (Smith et al., 1987; Bresink et al., 1995; Hirota et al., 2002). Furthermore, ketamine has been shown to inhibit norepinephrine and dopamine transporters (Nishimura et al., 1998b). Ketamine is only 20- to 60-fold more selective for the NMDA receptor over these other GPCRs and transporters, whereas MK-801 is more than 1500-fold more selective for NMDA receptor as compared to these other receptors. Thus, it is possible that ketamine is producing ‘off-target’ effects at one or more of these receptors.
4.4 NMDA antagonist effects on ICSS in the absence of the noxious stimulus
The present dissociation of MK-801 and ketamine effects on acid-induced depression of ICSS is also consistent with a dissociation in MK-801 and ketamine effects on ICSS in the absence of a noxious stimulus. Specifically, many drugs of abuse increase low ICSS rates maintained by low frequencies or intensities of brain stimulation, and this drug-induced ‘facilitation’ of ICSS is often interpreted as evidence of abuse potential (Carlezon and Chartoff, 2007;Negus and Miller, 2014). Both MK-801 and ketamine effects on ICSS have been evaluated in this context, and MK-801 is more likely than ketamine to produce ICSS facilitation (Corbett, 1989; Herberg and Rose, 1989; Carlezon and Wise, 1993; Bespalov et al., 1999; Hillhouse et al., 2014). For example, we reported previously that MK-801 doses of 0.1–0.18 mg/kg facilitated ICSS, whereas no ketamine dose from 1.0 to 10 mg/kg facilitated ICSS (Hillhouse et al., 2014). In the present study, the 0.18 mg/kg MK-801 dose was not tested, and other doses did not significantly facilitate ICSS; however, lower MK-801 doses did produce a trend towards ICSS facilitation in the present study (Fig. 3D–F), and these effects met criteria for statistical significance in our previous study. Conversely, ketamine failed to produce even a trend towards ICSS facilitation in either study. Although ketamine is designated as a Schedule III drug by the Drug Enforcement Agency and considered a drug of abuse, the illicit use of ketamine is small compared to other drugs of abuse like marijuana, nonprescription opioids and stimulants (Center for Behavioral Health Statistics and Quality, 2015). Additionally, social context appear to play a major role in the illicit use of ketamine. These results that ketamine does not facilitate ICSS, but is abuse in humans, parallel the ICSS results found with Δ9-tetrahydrocannabinol (Δ9-THC), which also fails to facilitate ICSS (Kwilasz and Negus, 2012). Overall, the present and previous studies are consistent with the conclusion that MK-801 can produce modest ICSS facilitation across a narrow range of intermediate doses, whereas ketamine does not.
5. Conclusion
In conclusion, this study adds to the growing body of literature examining the antinociceptive effects of NMDA receptor antagonists on pain-stimulated and pain-depressed behaviours. Specifically, the present study supports further consideration of NMDA receptor antagonists as analgesics; however, NMDA receptor antagonists with high affinity and selectivity for NMDA receptors (e.g. MK-801) may be more efficacious at attenuating pain-depressed behaviours as compared to NMDA receptor antagonists with lower affinity and selectivity (e.g. ketamine). Moreover, these results suggest that NMDA receptor antagonists like MK-801 can produce analgesic effects at doses that produce relatively weak evidence for abuse potential.
Acknowledgments
Funding sources
This research was supported by the National Institutes of Health under awards R01NS070715 (SSN) and T32DA07268 (TMH). The content is solely the responsibility of the authors and the National Institutes of Health had no other role than financial support.
Footnotes
Conflicts of interest
None declared.
Author contributions
Todd M. Hillhouse and S. Stevens Negus contributed to research design, data analysis and wrote the manuscript. Todd M. Hillhouse conducted the experiments. All authors discussed the results and commented on the manuscript.
References
- Altarifi AA, Rice KC, Negus SS. Effects of μ-opioid receptor agonists in assays of acute pain-stimulated and pain-depressed behavior in male rats: Role of μ-agonist efficacy and noxious stimulus intensity. J Pharmacol Exp Ther. 2015;352:208–217. doi: 10.1124/jpet.114.219873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amr YM. Multi-day low dose ketamine infusion as adjuvant to oral gabapentin in spinal cord injury related chronic pain: A prospective, randomized, double blind trial. Pain Physician. 2010;13:245–249. [PubMed] [Google Scholar]
- Andreasen JT, Bach A, Gynther M, Nasser A, Mogensen J, Strømgaard K, Pickering DS. UCCB01-125, a dimeric inhibitor of PSD-95, reduces inflammatory pain without disrupting cognitive or motor performance: Comparison with the NMDA receptor antagonist MK-801. Neuropharmacology. 2013;67:193–200. doi: 10.1016/j.neuropharm.2012.11.006. [DOI] [PubMed] [Google Scholar]
- Backonja M, Arndt G, Gombar KA, Check B, Zimmermann M. Response of chronic neuropathic pain syndromes to ketamine: A preliminary study. Pain. 1994;56:51–57. doi: 10.1016/0304-3959(94)90149-X. [DOI] [PubMed] [Google Scholar]
- Bespalov A, Lebedev A, Panchenko G, Zvartau E. Effects of abused drugs on thresholds and breaking points of intracranial self-stimulation in rats. Eur Neuropsychopharmacol. 1999;9:377–383. doi: 10.1016/s0924-977x(99)00008-5. [DOI] [PubMed] [Google Scholar]
- Bleakman D, Alt A, Nisenbaum ES. Glutamate receptors and pain. Semin Cell Dev Biol. 2006;17:592–604. doi: 10.1016/j.semcdb.2006.10.008. [DOI] [PubMed] [Google Scholar]
- Bresink I, Danysz W, Parsons CG, Mutschler E. Different binding affinities of NMDA receptor channel blockers in various brain regions—Indication of NMDA receptor heterogeneity. Neuropharmacology. 1995;34:533–540. doi: 10.1016/0028-3908(95)00017-z. [DOI] [PubMed] [Google Scholar]
- Bulutcu F, Dogrul A, Oguz Güç M. The involvement of nitric oxide in the analgesic effects of ketamine. Life Sci. 2002;71:841–853. doi: 10.1016/s0024-3205(02)01765-4. [DOI] [PubMed] [Google Scholar]
- Caldwell JR. Comparison of the efficacy, safety, and pharmacokinetic profiles of extended-release ketoprofen and piroxicam in patients with rheumatoid arthritis. Clin Ther. 1994;16:222–235. [PubMed] [Google Scholar]
- Carlezon WA, Chartoff EH. Intracranial self-stimulation (ICSS) in rodents to study the neurobiology of motivation. Nat Protocols. 2007;2:2987–2995. doi: 10.1038/nprot.2007.441. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Wise RA. Morphine-induced potentiation of brain stimulation reward is enhanced by MK-801. Brain Res. 1993;620:339–342. doi: 10.1016/0006-8993(93)90177-o. [DOI] [PubMed] [Google Scholar]
- Center for Behavioral Health Statistics and Quality. Behavioral Health Trends in the United States: Results from the 2014 National Survey on Drug Use and Health. 2015. (HHS Publication No. SMA 15-4927, NSDUH Series H-50). [Google Scholar]
- Christoph T, Schiene K, Englberger W, Parsons CG, Chizh BA. The antiallodynic effect of NMDA antagonists in neuropathic pain outlasts the duration of the in vivo NMDA antagonism. Neuropharmacology. 2006;51:12–17. doi: 10.1016/j.neuropharm.2006.02.007. [DOI] [PubMed] [Google Scholar]
- Cleeland CS, Ryan KM. Pain assessment: Global use of the Brief Pain Inventory. Ann Acad Med Singapore. 1994;23:129–138. [PubMed] [Google Scholar]
- Corbett D. Possible abuse potential of the NMDA antagonist MK-801. Behav Brain Res. 1989;34:239–246. doi: 10.1016/s0166-4328(89)80105-6. [DOI] [PubMed] [Google Scholar]
- Felsby S, Nielsen J, Arendt-Nielsen L, Jensen TS. NMDA receptor blockade in chronic neuropathic pain: A comparison of ketamine and magnesium chloride. Pain. 1996;64:283–291. doi: 10.1016/0304-3959(95)00113-1. [DOI] [PubMed] [Google Scholar]
- Finck AD, Samaniego E, Ngai SH. Morphine tolerance decreases the analgesic effects of ketamine in mice. Anesthesiology. 1988;68:397–400. doi: 10.1097/00000542-198803000-00013. [DOI] [PubMed] [Google Scholar]
- Fundytus ME. Glutamate receptors and nociception: Implications for the drug treatment of pain. CNS Drugs. 2001;15:29–58. doi: 10.2165/00023210-200115010-00004. [DOI] [PubMed] [Google Scholar]
- Gilmour G, Pioli E, Dix S, Smith J, Conway M, Jones W, Loomis S, Mason R, Shahabi S, Tricklebank M. Diverse and often opposite behavioural effects of NMDA receptor antagonists in rats: Implications for “NMDA antagonist modelling” of schizophrenia. Psychopharmacology. 2009;205:203–216. doi: 10.1007/s00213-009-1530-7. [DOI] [PubMed] [Google Scholar]
- Herberg LJ, Rose IC. The effect of MK-801 and other antagonists of NMDA-type glutamate receptors on brain-stimulation reward. Psycho-pharmacology. 1989;99:87–90. doi: 10.1007/BF00634458. [DOI] [PubMed] [Google Scholar]
- Hillhouse TM, Porter JH. Ketamine, but not MK-801, produces antidepressant-like effects in rats responding on a differential-reinforcement-of-low-rate operant schedule. Behav Pharmacol. 2014;25:80–91. doi: 10.1097/FBP.0000000000000014. [DOI] [PubMed] [Google Scholar]
- Hillhouse T, Porter J, Negus SS. Dissociable effects of the noncompetitive NMDA receptor antagonists ketamine and MK-801 on intracranial self-stimulation in rats. Psychopharmacology. 2014;231:2705–2716. doi: 10.1007/s00213-014-3451-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota K, Hashimoto Y, Lambert DG. Interaction of intravenous anesthetics with recombinant human M1–M3 muscarinic receptors expressed in Chinese hamster ovary cells. Anest Analg. 2002;95:1607–1610. doi: 10.1097/00000539-200212000-00025. [DOI] [PubMed] [Google Scholar]
- Jahangiri L, Kesmati M, Najafzadeh H. Evaluation of analgesic and anti-inflammatory effect of nanoparticles of magnesium oxide in mice with and without ketamine. Eur Rev Med Pharmacol Sci. 2013;17:2706–2710. [PubMed] [Google Scholar]
- Jørum E, Warncke T, Stubhaug A. Cold allodynia and hyperalgesia in neuropathic pain: The effect of N-methyl-D-aspartate (NMDA) receptor antagonist ketamine – A double-blind, cross-over comparison with alfentanil and placebo. Pain. 2003;101:229–235. doi: 10.1016/S0304-3959(02)00122-7. [DOI] [PubMed] [Google Scholar]
- Kim K, Mishina M, Kokubo R, Nakajima T, Morimoto D, Isu T, Kobayashi S, Teramoto A. Ketamine for acute neuropathic pain in patients with spinal cord injury. J Clin Neurosci. 2013;20:804–807. doi: 10.1016/j.jocn.2012.07.009. [DOI] [PubMed] [Google Scholar]
- Kokki H, Homan E, Tuovinen K, Purhonen S. Peroperative treatment with i.v. ketoprofen reduces pain and vomiting in children after strabismus surgery. Acta Anaesthesiol Scand. 1999;43:13–18. doi: 10.1034/j.1399-6576.1999.430104.x. [DOI] [PubMed] [Google Scholar]
- Kuiken S, Berg STV, Tytgat GJ, Boeckxstaens GE. Oral S (+)-ketamine does not change visceral perception in health. Dig Dis Sci. 2004;49:1745–1751. doi: 10.1007/s10620-004-9563-6. [DOI] [PubMed] [Google Scholar]
- Kwilasz AJ, Negus SS. Dissociable effects of the cannabinoid receptor agonists Δ9-Tetrahydrocannabinol and CP55940 on pain-stimulated versus pain-depressed behavior in rats. J Pharmacol Exp Ther. 2012;343:389–400. doi: 10.1124/jpet.112.197780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwilasz AJ, Abdullah RA, Poklis JL, Lichtman AH, Negus SS. Effects of the fatty acid amide hydrolase inhibitor URB597 on pain-stimulated and pain-depressed behavior in rats. Behav Pharmacol. 2014;25:119–129. doi: 10.1097/FBP.0000000000000023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitl MD, Onvani S, Bowers MS, Cheng K, Rice KC, Carlezon WA, Jr, Banks ML, Negus SS. Pain-related depression of the mesolimbic dopamine system in rats: Expression, blockade by analgesics, and role of endogenous [kappa]-opioids. Neuropsychopharmacology. 2014;39:614–624. doi: 10.1038/npp.2013.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung A, Wallace MS, Ridgeway B, Yaksh T. Concentration-effect relationship of intravenous alfentanil and ketamine on peripheral neurosensory thresholds, allodynia and hyperalgesia of neuropathic pain. Pain. 2001;91:177–187. doi: 10.1016/s0304-3959(00)00433-4. [DOI] [PubMed] [Google Scholar]
- Malec D, Poleszak E. Adenosine receptor ligands and dizocilpineinduced antinociception in mice. Int J Neurosci. 2005;115:511–522. doi: 10.1080/00207450590519139. [DOI] [PubMed] [Google Scholar]
- McMillan DE, Wright DW, Wenger GR. Effects of phencyclidine-like drugs on responding under multiple fixed ratio, fixed interval schedules. Behav Pharmacol. 1992;3:143–147. [PubMed] [Google Scholar]
- Millan MJ, Seguin L. Chemically-diverse ligands at the glycine B site coupled to N-methyl-D-aspartate (NMDA) receptors selectively block the late phase of formalin-induced pain in mice. Neurosci Lett. 1994;178:139–143. doi: 10.1016/0304-3940(94)90309-3. [DOI] [PubMed] [Google Scholar]
- Miller LL, Altarifi AA, Negus SS. Effects of repeated morphine on intracranial self-stimulation in male rats in the absence or presence of a noxious pain stimulus. Exp Clin Psychopharmacol. 2015a;23:405–414. doi: 10.1037/pha0000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller LL, Leitl MD, Banks ML, Blough BE, Negus SS. Effects of the triple monoamine uptake inhibitor amitifadine on pain-related depression of behavior and mesolimbic dopamine release in rats. Pain. 2015b;156:175–184. doi: 10.1016/j.pain.0000000000000018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS. Expression and treatment of pain-related behavioral depression. Lab Animal. 2013;42:292+. doi: 10.1038/laban.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS, Miller LL. Intracranial self-stimulation to evaluate abuse potential of drugs. Pharmacol Rev. 2014;66:869–917. doi: 10.1124/pr.112.007419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS, Bilsky E, Carmo G, Stevenson G. Rationale and methods for assessment of pain-depressed behavior in preclinical assays of pain and analgesia. In: Szallasi A, editor. Analgesia. Humana Press; 2010a. pp. 79–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS, Morrissey E, Rosenberg M, Cheng K, Rice K. Effects of kappa opioids in an assay of pain-depressed intracranial self-stimulation in rats. Psychopharmacology. 2010b;210:149–159. doi: 10.1007/s00213-009-1770-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS, O’Connell R, Morrissey E, Cheng K, Rice KC. Effects of peripherally restricted κ opioid receptor agonists on pain-related stimulation and depression of behavior in rats. J Pharmacol Exp Ther. 2012a;340:501–509. doi: 10.1124/jpet.111.186783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS, Rosenberg MB, Altarifi AA, O’Connell RH, Folk JE, Rice KC. Effects of the delta opioid receptor agonist SNC80 on pain-related depression of intracranial self-stimulation (ICSS) in rats. J Pain. 2012b;13:317–327. doi: 10.1016/j.jpain.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niesters M, Dahan A. Pharmacokinetic and pharmacodynamic considerations for NMDA receptor antagonists in the treatment of chronic neuropathic pain. Expert Opin Drug Metab Toxicol. 2012;8:1409–1417. doi: 10.1517/17425255.2012.712686. [DOI] [PubMed] [Google Scholar]
- Niesters M, Martini C, Dahan A. Ketamine for chronic pain: Risks and benefits. Br J Clin Pharmacol. 2014;77:357–367. doi: 10.1111/bcp.12094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura M, Sato K, Okada T, Schloss P, Shimada S, Tohyama M. MK-801 blocks monoamine transporters expressed in HEK cells. FEBS Lett. 1998a;423:376–380. doi: 10.1016/s0014-5793(98)00126-4. [DOI] [PubMed] [Google Scholar]
- Nishimura M, Sato K, Okada T, Yoshiya I, Schloss P, Shimada S, Tohyama M. Ketamine inhibits monoamine transporters expressed in human embryonic kidney 293 cells. Anesthesiology. 1998b;88:768–774. doi: 10.1097/00000542-199803000-00029. [DOI] [PubMed] [Google Scholar]
- Pereira Do Carmo G, Stevenson GW, Carlezon WA, Negus SS. Effects of pain- and analgesia-related manipulations on intracranial self-stimulation in rats: Further studies on pain-depressed behavior. Pain. 2009;144:170–177. doi: 10.1016/j.pain.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg MB, Carroll FI, Negus SS. Effects of monoamine reuptake inhibitors in assays of acute pain-stimulated and pain-depressed behavior in rats. J Pain. 2013;14:246–259. doi: 10.1016/j.jpain.2012.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryder S, Way WL, Trevor AJ. Comparative pharmacology of the optical isomers of ketamine in mice. Eur J Pharmacol. 1978;49:15–23. doi: 10.1016/0014-2999(78)90217-0. [DOI] [PubMed] [Google Scholar]
- Sabetkasaie M, Khansefid N, Ladgevardi MARS. Possible role of NMDA receptors in antinociception induced by rilmenidine in mice in the formalin test. Eur J Pain. 2007;11:535–541. doi: 10.1016/j.ejpain.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Sawynok J. Topical and peripheral ketamine as an analgesic. Anest Analg. 2014;119:170–178. doi: 10.1213/ANE.0000000000000246. [DOI] [PubMed] [Google Scholar]
- Sawynok J, Reid A. Modulation of formalin-induced behaviors and edema by local and systemic administration of dextromethorphan, memantine and ketamine. Eur J Pharmacol. 2002;450:153–162. doi: 10.1016/s0014-2999(02)02119-2. [DOI] [PubMed] [Google Scholar]
- Schwartzman RJ, Alexander GM, Grothusen JR, Paylor T, Reichenberger E, Perreault M. Outpatient intravenous ketamine for the treatment of complex regional pain syndrome: A double-blind placebo controlled study. Pain. 2009;147:107–115. doi: 10.1016/j.pain.2009.08.015. [DOI] [PubMed] [Google Scholar]
- Seguin L, Le Marouille-Girardon S, Millan MJ. Antinociceptive profiles of non-peptidergic neurokinin1 and neurokinin2 receptor antagonists: A comparison to other classes of antinociceptive agent. Pain. 1995;61:325–343. doi: 10.1016/0304-3959(94)00194-J. [DOI] [PubMed] [Google Scholar]
- Sigtermans MJ, van Hilten JJ, Bauer MCR, Arbous MS, Marinus J, Sarton EY, Dahan A. Ketamine produces effective and long-term pain relief in patients with Complex Regional Pain Syndrome Type 1. Pain. 2009;145:304–311. doi: 10.1016/j.pain.2009.06.023. [DOI] [PubMed] [Google Scholar]
- Smith DJ, Bouchal RL, DeSanctis CA, Monroe PJ, Amedro JB, Perrotti JM, Crisp T. Properties of the interaction between ketamine and opiate binding sites in vivo and in vitro. Neuropharmacology. 1987;26:1253–1260. doi: 10.1016/0028-3908(87)90084-0. [DOI] [PubMed] [Google Scholar]
- Smith J, Gastambide F, Gilmour G, Dix S, Foss J, Lloyd K, Malik N, Tricklebank M. A comparison of the effects of ketamine and phencyclidine with other antagonists of the NMDA receptor in rodent assays of attention and working memory. Psychopharmacology. 2011;217:255–269. doi: 10.1007/s00213-011-2277-5. [DOI] [PubMed] [Google Scholar]
- Suardíaz M, Estivill-Torrús G, Goicoechea C, Bilbao A, Rodriguez de Fonseca F. Analgesic properties of oleoylethanolamide (OEA) in visceral and inflammatory pain. Pain. 2007;133:99–110. doi: 10.1016/j.pain.2007.03.008. [DOI] [PubMed] [Google Scholar]
- Swartjes M, Morariu A, Niesters M, Aarts L, Dahan A. Nonselective and NR2B-selective N-methyl-D-aspartic acid receptor antagonists produce antinocicpetion and long-term relief of allodynia in acute and neuropathic pain. Anesthesiology. 2011;115:165–174. doi: 10.1097/ALN.0b013e31821bdb9b. [DOI] [PubMed] [Google Scholar]
- Takahashi RN, Morato GS, Rae GA. Effects of ketamine on nociception and gastrointestinal motility in mice are unaffected by naloxone. Gen Pharmacol. 1987;18:201–203. doi: 10.1016/0306-3623(87)90251-5. [DOI] [PubMed] [Google Scholar]
- Turk DC, Dworkin RH, McDermott MP, Bellamy N, Burke LB, Chandler JM, Cleeland CS, Cowan P, Dimitrova R, Farrar JT, Hertz S, Heyse JF, Iyengar S, Jadad AR, Jay GW, Jermano JA, Katz NP, Manning DC, Martin S, Max MB, McGrath P, McQuay HJ, Quessy S, Rappaport BA, Revicki DA, Rothman M, Stauffer JW, Svensson O, White RE, Witter Jz. Analyzing multiple endpoints in clinical trials of pain treatments: IMMPACT recommendations. Pain. 2008;139:485–493. doi: 10.1016/j.pain.2008.06.025. [DOI] [PubMed] [Google Scholar]
- Veys EM. 20 years’ experience with ketoprofen. Scand J Rheumatol Suppl. 1991;90:1–44. [PubMed] [Google Scholar]
- Wang J, Goffer Y, Xu D, Tukey DS, Shamir DB, Eberle SE, Zou AH, Blanck TJ, Ziff EB. A single subanesthic dose of ketamine relieves depression-like behaviors induced by neuropathic pain in rats. Anesthesiology. 2011;115:812–821. doi: 10.1097/ALN.0b013e31822f16ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozniak KM, Rojas C, Wu Y, Slusher BS. The role of glutamate signaling in pain processes and its regulation by GCP II inhibition. Curr Med Chem. 2012;19:1323–1334. doi: 10.2174/092986712799462630. [DOI] [PubMed] [Google Scholar]


