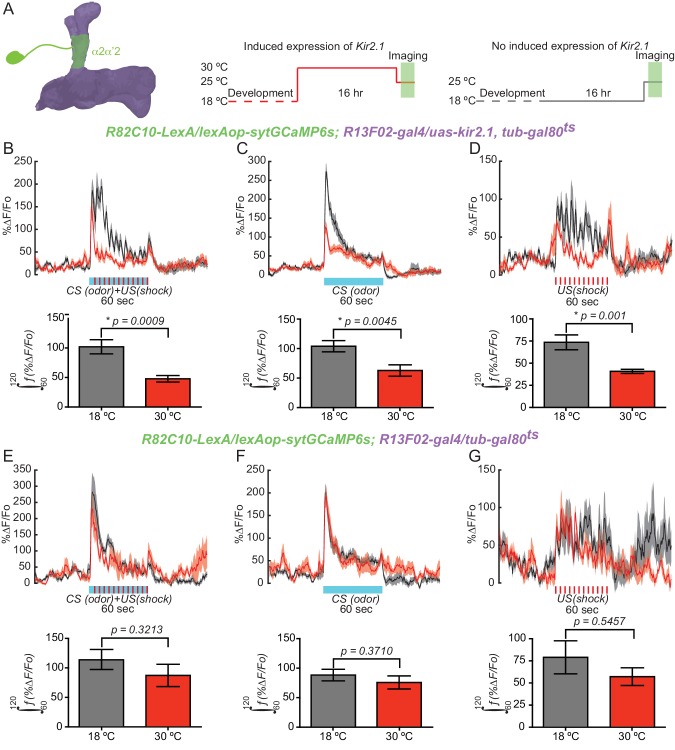
Figure 4. KC input is required for normal DAn synaptic release during learning.
(A) Diagram of the experimental setup. Flies of the indicated genotype were reared at 18°C and then 1–2 day old adults were switched to 30°C to induce kir2.1 channel expression in KC for 16 hr. Flies were then returned to room temperature and prepared for functional imaging. Control flies remained at 18°C until removing them to room temperature for functional imaging. (B) Calcium responses in α2α’2 DAn during aversive olfactory training (odor+shock) in flies with (red) or without (black) kir2.1 expression in KC. N = 10–12. The solid line represents the mean and the shaded area represents ± SEM. Data were analyzed using Mann-Whitney U non-parametric test. Bars represent the mean ± SEM of the area under the curve during the one minute of conditioning (the 60–120 s imaging time window). (C) Calcium responses in α2α’2 DAn during odor delivery in flies with (red) or without (black) kir2.1 expression in KC. N = 13–14. The solid line represents the mean and the shaded area represents ± SEM. Data were analyzed using Mann-Whitney U non-parametric test. Bars represent the mean ± SEM of the area under the curve during the one minute of odor presentation (the 60–120 s imaging time windows). (D) Calcium responses in α2α’2 DAn during 12 shock pulses delivery in flies with (red) or without (black) kir2.1 expression in KC. N = 14. The solid line represents the mean and the shaded area represents ± SEM. Data were analyzed using Mann-Whitney U non-parametric test. Bars represent the mean ± SEM of the area under the curve during the one minute of shock pulses (the 60–120 s imaging time window) (E–G) Parallel data to panels B–D but using flies without the kir2.1 transgene. These control data show that the differential responses observed in panels B–D require the kir2.1 expression.