Abstract
The atrial-specific ultrarapid delayed rectifier K+ current (IKur) inactivates slowly but completely at depolarized voltages. The consequences for IKur rate-dependence have not been analyzed in detail and currently available mathematical action-potential (AP) models do not take into account experimentally observed IKur inactivation dynamics. Here, we developed an updated formulation of IKur inactivation that accurately reproduces time-, voltage-, and frequency-dependent inactivation. We then modified the human atrial cardiomyocyte Courtemanche AP model to incorporate realistic IKur inactivation properties. Despite markedly different inactivation dynamics, there was no difference in AP parameters across a wide range of stimulation frequencies between the original and updated models. Using the updated model, we showed that, under physiological stimulation conditions, IKur does not inactivate significantly even at high atrial rates because the transmembrane potential spends little time at voltages associated with inactivation. Thus, channel dynamics are determined principally by activation kinetics. IKur magnitude decreases at higher rates because of AP changes that reduce IKur activation. Nevertheless, the relative contribution of IKur to AP repolarization increases at higher frequencies because of reduced activation of the rapid delayed-rectifier current IKr. Consequently, IKur block produces dose-dependent termination of simulated atrial fibrillation (AF) in the absence of AF-induced electrical remodeling. The inclusion of AF-related ionic remodeling stabilizes simulated AF and greatly reduces the predicted antiarrhythmic efficacy of IKur block. Our results explain a range of experimental observations, including recently reported positive rate-dependent IKur-blocking effects on human atrial APs, and provide insights relevant to the potential value of IKur as an antiarrhythmic target for the treatment of AF.
Introduction
The ultrarapid delayed rectifier K+ current (IKur) was originally described as a rapidly activating, 4-aminopyridine-sensitive K+ current with slow and limited inactivation (1). However, many of the original experiments used relatively short test pulses, typically <4 s long, which may have not been enough to observe the full extent of IKur’s inactivation. Feng et al. (2), using longer 50-s test pulses, showed that IKur inactivates almost completely when the cell is depolarized to +40 mV, with a biexponential time course comprising fast (τf) and slow time constants (τs) of 1.0 and 6.8 s, respectively. Based on these experiments, it has been hypothesized that IKur inactivation may play an important role under physiological conditions, specifically related to IKur frequency-dependent dynamics (2). The potential for full inactivation of IKur suggests that the contribution of the current would decrease at faster rates, as the total amount of time when the cell is depolarized increases and inactivation accumulates. However, recent experimental results using the IKur-selective blocker XEN-D0103 suggest the opposite, and indicate that the contribution of IKur to atrial repolarization increases at higher rates (3).
To our knowledge, none of the commonly used mathematical models of the human atrial cardiomyocyte action potential (AP) integrate the experimentally defined IKur inactivation dynamics reported by Feng et al. (2). To better understand the rate-dependent role of IKur and the effect of blocking it on the human atrial AP, it is necessary to use a model with accurate inactivation properties. In this study, we sought to 1) quantify the kinetic features of the IKur inactivation properties defined by Feng et al. (2); 2) update the Courtemanche human atrial AP model (4) to include realistic IKur inactivation parameters; 3) investigate the rate-dependent properties of IKur inactivation over the course of the AP under physiological conditions; 4) evaluate the contribution of IKur with realistic inactivation properties to human atrial repolarization at different frequencies; and 5) evaluate the potential consequences for effects of blocking IKur on atrial fibrillation (AF) in non-remodeled and AF-remodeled atrial tissue.
Materials and Methods
Mathematical modeling of IKur inactivation
The experimental data recorded by Feng et al. (2) were used as the basis for model development in our study. A description of the experimental methods can be found in the original article (2). The Courtemanche human atrial cardiomyocyte model was implemented (4). The original formulation of the IKur inactivation parameter is as follows:
where IKur is the ultrarapid delayed rectifier K+ current, gKur is the maximal IKur conductance, V is the transmembrane potential, EK is the K+ equilibrium potential, and ua and ui are IKur’s activation and inactivation gating variables, respectively; αui and βui are the forward and backward rate constants for the inactivation gate variable, respectively; τui is the time constant; ui,∞ is the steady-state relation for the inactivation gate variable; Cm is the membrane capacitance (100 pF); and KQ10 is a scaling factor (3).
To account for IKur inactivation dynamics we replaced the original inactivation gating variable ui by fast (ui,f) and slow (ui,s) inactivation gating variables. The gating variables and time constant parameters were fitted by iteratively minimizing the mean error of the model time constants relative to experimentally derived values obtained with a 50-s test-pulse at 0, 10, 20, 30, 40, and 50 mV (Fig. S1, B and C); the modified inactivation gating variables and time constants are given by the following:
The activation gating variable and time constant were not modified and are given by the following:
The activation gate open probability is given by ua3. The inactivation gate open probability in the original model was given by ui; in the modified model ui,s × ui,f was used to reflect the combined effects of slow and fast inactivation components. Open probabilities range from 0 (closed) to 1 (open). The charge carried by IKur in one cardiac cycle, given by the area under the IKur-versus-time curve (Kur AUC), was calculated by integrating IKur over one cardiac cycle. The activation gate open-probability area under the curve (ua3 AUC) was calculated by integrating ua3 over one cardiac cycle. The AP amplitude was defined as the difference between the peak phase-0 overshoot potential and the resting membrane potential.
Mathematical simulations
The Courtemanche model of human atrial cardiomyocyte bioelectricity was implemented with the original and updated IKur inactivation formulations. The total ionic current for the model (Itot) was given by the following:
where INa is the fast inward Na+ current; IK1 is the inward rectifier K+ current; Ito is the transient outward K+ current; IKur, IKr, and IKs are the ultrarapid, rapid, and slow components of the delayed rectifier K+ current, respectively; ICa,L is the L-type inward Ca2+ current; Ip,Ca is the sarcolemmal Ca2+ pump current; INaK is the Na+/K+ pump current; INaCa is the Na+/Ca2+ exchanger current; Ib,Na is the background Na+ current; Ib,Ca is the background Ca2+ current; and IK,ACh is the acetylcholine-activated K+ current (5). Isolated cardiomyocyte APs were simulated at 37°C by numerical integration with the software MATLAB’s ODE23s ordinary differential equation solver (The MathWorks, Natick, MA) with a relative tolerance of 10−2. For each condition, pacing was sustained for 280 cycles with 2-ms 3 nA stimuli and the last action potential was used for analysis.
Computer simulations of the effect of IKur block on AF were performed with the CARP simulator, which solves the monodomain equation by the finite element method as described previously (6), as follows:
where σi is the tissue conductivity, β is the membrane surface-to-volume ratio (0.14 μm−1), and Cm is the membrane capacitance (1 μF/cm2). The updated Courtemanche ionic model was adapted and implemented in CARP. Conductivities were chosen to give a physiological conduction velocity in the longitudinal direction (47.9 cm/s). The tissue measured 7 × 6 cm with fibers oriented along the long dimension with an anisotropy ratio of ∼6. The grid was discretized at 100-μm resolution and equations solved with a 25-μs timestep. Reentry was initiated by a standard S1-S2 cross-shock protocol with an S1-S2 interval of 170 ms.
Two-dimensional simulations were conducted using three different acetylcholine (ACh) distribution patterns (one homogeneous ACh distribution and two sinusoidal distributions) and three peak ACh concentrations (1.875, 3.75, and 7.5 nM), generating nine conditions. IKur block was simulated by a fixed reduction in maximal IKur conductance (gKur). Dose-response curves were generated by introducing IKur block at 10 different time-points {tdrug = 1000 ms, 1100 ms, … 1800 ms, 1900 ms} for each percent IKur block {10%, 20%, …, 90%, 100%}; the average time to termination was quantified as the time from tdrug to reentry termination. Simulations were run using the control (non-remodeled) Courtemanche human atrial AP model and repeated with an AF-remodeled AP model consisting of the control model with the following modifications: Ito conductance reduced by 50%, IKur conductance reduced by 50%, ICa,L conductance reduced by 70%, and IK1 conductance increased by 100% (7).
Results
Experimentally observed time-dependent inactivation of IKur
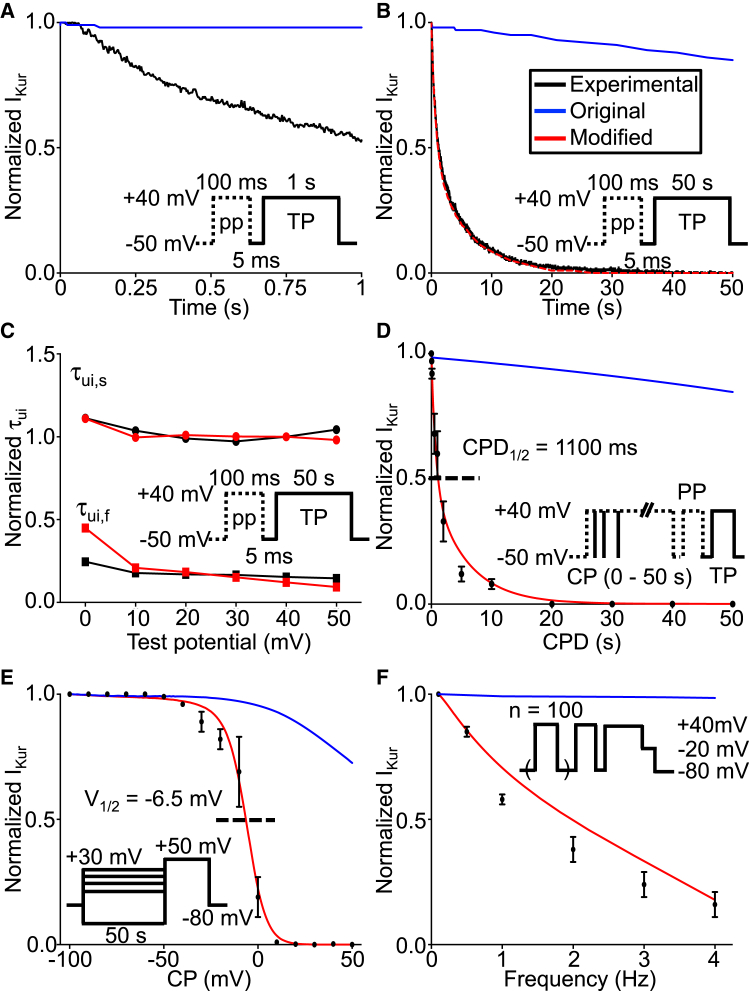
The ultrarapid delayed rectifier K+ current (IKur) is often described as having slow and partial inactivation. Fig. 1 A shows normalized IKur during a 1000 ms test-pulse to +40 mV, 5 ms after a 100-ms pretest pulse to +40 mV to inactivate Ito, as recorded experimentally (black) (2) and from the original Courtemanche human atrial IKur formulation (blue). Using this relatively short test pulse, experimental IKur inactivates by ∼45% (black). In contrast to experimental data, in the original Courtemanche model, IKur does not inactivate appreciably over the course of the pulse (blue). Fig. 1 B shows normalized IKur during a longer, 50 s test-pulse to +40 mV, 5 ms after a 100-ms pretest pulse to +40 mV to inactivate Ito. With the longer pulse, the experimentally recorded IKur (black) inactivated completely with a biexponential time-course characterized by fast and slow time constants of 702 ± 7 and 5688 ± 26 ms, respectively. Again, the original Courtemanche model (blue) fails to reproduce IKur’s inactivation dynamics, with 85% of the peak current persisting at the end of the 50-s test-pulse.
Figure 1.
Time-, voltage-, and frequency dependence of IKur inactivation. (A) Shown here is the normalized IKur current during a 1000-ms test pulse (TP) at +40 mV, 5 ms after a 100-ms prepulse (PP), to inactivate Ito (inset) as recorded from the experimental preparation (black) and the original Courtemanche human atrial model (blue). (B) Normalized IKur current using a similar protocol, but with test pulse duration of 50 s as recorded from the experimental preparation (black) and the original and modified Courtemanche model (blue and red, respectively), is given. The experimental and modified model time constants are τui,f,exp = 702 ± 7 ms, τui,s,exp = 5688 ± 26 ms and τui,f,model = 713 ± 18 ms, τui,s,model = 5848 ± 188 ms, respectively. (C) Normalized fast (τui,f) and slow (τui,s) time constants as a function of test pulse potential for the experimental preparation and the modified Courtemanche model at 37°C are shown. (D) Shown here is the normalized IKur current using a CPD to +40 mV, followed by a 100-ms prepulse (PP) to +40 mV to inactivate Ito preceding a 100-ms test pulse (TP) to +40 mV (inset), as recorded from the experimental preparation and the original and modified models; the half-inactivation CPD (CPD1/2) was 1100 ms. (E) Shown here is the normalized IKur current using a 50 s-conditioning pulse (CP) to various voltages followed by a 240-ms test pulse to +50 mV (inset); the half-inactivation voltage (V1/2) was –6.5 mV in the model and –7.5 ± 0.6 mV experimentally. (F) Shown here is the normalized IKur current obtained by applying 100 pretest stimuli of 100 ms duration at +40 mV at various frequencies, followed by a 100-ms conditioning pulse to +40 mV to inactivate Ito and a 140-ms test pulse to +40 mV (inset); the normalized current at 4 Hz was 16% of its value at 0.1 Hz. For all panels, experimental data are in black; original and modified models are in blue and red, respectively.
Model inactivation kinetics modified according to experimental data
To accurately reproduce the experimentally recorded IKur inactivation kinetics, we modified the Courtemanche model by introducing a set of slow (ui,s) and fast (ui,f) inactivation gating variables in place of the single inactivation gating variable (ui) in the original model. The voltage dependence of the inactivation gating variables for the original (ui,original, blue line) and modified (ui,f, red solid line; ui,s, red dashed line) models is shown in Fig. S1 A. The major modification to inactivation gating is more complete inactivation at depolarized potentials; the complete mathematical formulation can be found in Materials and Methods. We also replaced the inactivation time constant (τui,original) with a set of fast (τui,f) and slow (τui,s) inactivation time constants (Fig. S1 B). Note that the updated inactivation time constants are of the order of 1–6 s. The voltage dependence of the activation gating variable (Fig. S1 C; ua,x) and activation time constant (Fig. S1 D, τua,x) were not modified. The activation time constant is in the range of 1–6 ms.
The modified model (red line) closely reproduced the experimentally recorded current inactivation (black line) during a prolonged pulse (Fig. 1 B) and the experimentally recorded IKur inactivation kinetics (Fig. 1 C) at 37°C. Excellent agreement between experiments and the mathematical model was obtained across the spectrum of test potentials.
Time-, voltage-, and frequency dependence of IKur inactivation
We then compared the time dependence of IKur inactivation in the model to experimental results obtained by varying the duration of the test pulse. Fig. 1 D (black) shows experimentally recorded normalized IKur obtained with conditioning pulses to +40 mV of varying durations, followed by a 100-ms prepulse to +40 mV to inactivate Ito and then a 100-ms test pulse to +40 mV. IKur displayed complete inactivation with conditioning pulses >20 s. In contrast, the original Courtemanche model-derived results showed little inactivation even with the longest conditioning-pulse duration (Fig. 1 D, blue). Conversely, the modified model closely replicated the experimental data (Fig. 1 D, red); the half-inactivation conditioning pulse duration (CPD1/2) was 1100 ms at +40 mV. The voltage dependence of IKur inactivation was then assessed by applying a 50 s pretest pulse to various voltages followed by a 240-ms test pulse to +50 mV; the experimentally recorded normalized IKur is shown in Fig. 1 E (black). IKur inactivation is highly dependent on the pretest pulse potential. The original Courtemanche model (blue) did not reproduce the experimentally observed voltage-dependent inactivation dependence (Fig. 1 E, black), whereas the modified model-generated results (red) closely matched experimental findings. The experimental and modified-model half-inactivation voltages (V1/2s) were −7.5 ± 0.6 and −6.5 mV, respectively.
The frequency dependence of IKur inactivation was studied by applying 100 pulses of 100-ms duration to +40 mV at various frequencies, followed by a 100-ms conditioning pulse to +40 mV to inactivate Ito and a 140-ms test pulse to +40 mV. Experimentally, IKur displayed marked frequency dependence with 84% reduction at 4 vs. 0.1 Hz (Fig. 1 F, black data). In contrast, the original Courtemanche model showed virtually no frequency dependence (Fig. 1 F, blue). The modified model was much more consistent with the experimental findings (Fig. 1 F, red).
Incorporation of realistic IKur inactivation kinetics and AP dynamics
Fig. S2 shows the AP amplitude (Fig. S2 A), AP duration at 90% repolarization (APD90, Fig. S2 B), transmembrane potential at the time of maximum phase 0 overshoot (overshoot potential, Fig. S2 C), and phase-0 peak Na+ current (peak INa, Fig. S2 D) as a function of cycle length (CL) for the original (black) and modified (red) Courtemanche models. Despite markedly different IKur inactivation kinetics, there were no significant differences in AP or Na+-current dynamics between the two models across a wide range of physiologically relevant cycle lengths. We then pursued the rate-dependent properties of IKur to understand why, despite much greater inactivation for both experimental and model pulse protocols than in the original Courtemanche model, there was no apparent effect on rate-dependent AP properties.
IKur activation and inactivation dynamics and mechanisms of rate-dependence
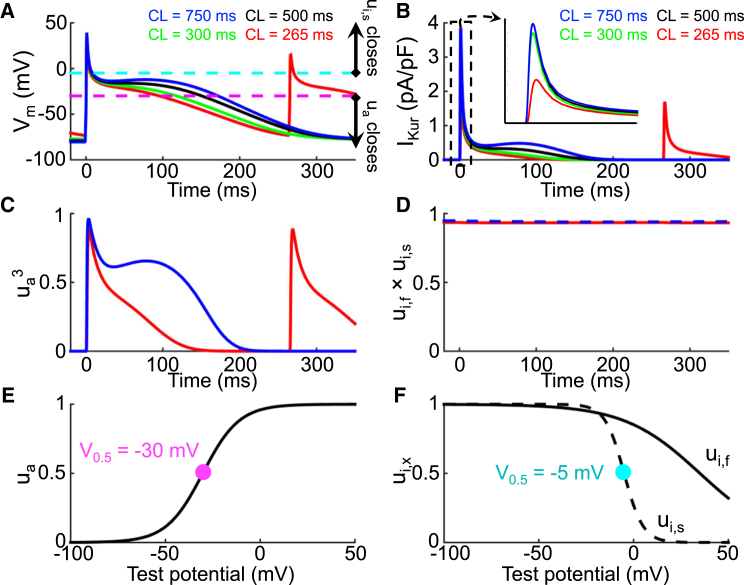
Fig. 2 A shows model APs at progressively shorter stimulation cycle lengths (750, 500, 300, and 265 ms). The AP duration is shortened and plateau potential becomes less positive as the dome disappears when stimulation cycle length decreases. Fig. 2 B shows the corresponding IKur simulations. There is a rate-dependent decrease in IKur during phase 2 of the AP with little change in peak IKur (inset) except at the shortest cycle length (inset, red). Fig. 2, C and D, shows the corresponding activation and inactivation gate open probabilities (ua3; ui,f × ui,s) as a function of time for the shortest (red) and longest (blue) cycle length. IKur activation decreases during phase 2 in a rate-dependent manner (Fig. 2 C), whereas inactivation is virtually rate independent (Fig. 2 D). Therefore, IKur rate dependence is driven by its activation gate kinetics, with little contribution from inactivation. This is further detailed in Fig. S3 where the rate dependence of the total IKur charge carried per cycle (Fig. S3 B) is shown to be due to changes in rate-dependent activation-gate open probability (Fig. S3 F), with no significant contribution from inactivation (Fig. S3 D).
Figure 2.
Mechanism of IKur rate dependence. (A) Action potentials at stimulation cycle lengths from 265 ms (red), 300 ms (green), 500 ms (black), and 750 ms (blue) are shown. The purple and teal dashed lines correspond to the activation and inactivation gating variable 50% (V0.5,ua = −30 mV and V0.5,uis = −5 mV) opening potentials, respectively. (B) Shown here are corresponding IKur tracings; there is a rate-dependent decrease in IKur during phase 2 of the AP. (C) Shown here is the activation gate open probability (ua3) and (D) inactivation gate open probability (ui,f × ui,s) as a function of time for CLs of 265 ms (red) and 750 ms (blue). The activation open probability is rate dependent and the inactivation open probability is rate independent. (E) Shown here is the activation gating variable (ua) as a function of transmembrane potential; the purple dot corresponds to activation gating variable V0.5 as transposed on (A). (F) Fast (ui,f; black solid) and slow (ui,s; black dashed) inactivation gating variables as a function of transmembrane potential are given; the teal dot corresponds to the slow inactivation gating variable V0.5 as transposed on (A). The inactivation open probability is rate independent because the action potential spends very little time positive to V0.5,uis (−5 mV; teal).
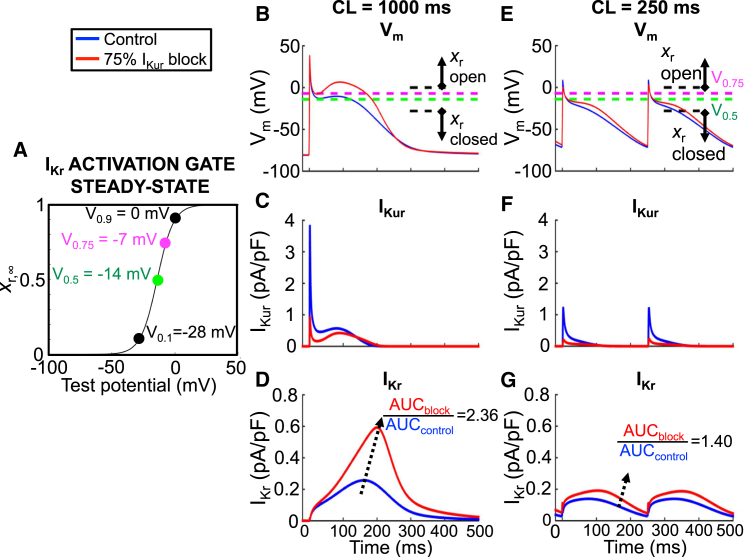
Figure 3.
Rate-dependent effects of IKur block on the AP. (A) Shown here is the IKr activation gate steady state (xr,∞) as a function of test potential. V0.1, V0.5, V0.75, and V0.9 are marked and transposed onto (B) and (E) as dashed lines. (B) Shown here are APs obtained at a cycle length of 1000 ms under control (blue) and with 75% IKur blockade (red); the AP duration at −60 mV (APD−60) was 255 ms for both. (C and D) Shown here are corresponding IKur and IKr tracings; the ratio of IKr with 75% IKur block to control was 2.36. (E) Shown here are APs obtained at a cycle length of 250 ms under control (blue) and with 75% IKur blockade (red); the APD−60 for control and 75% IKur block was 199 and 209 ms, respectively. (F and G) Shown here are corresponding IKur and IKr tracings; the ratio of IKr with 75% IKur block to control was 1.40.
Fig. 2 E shows the steady-state activation gating variable as a function of transmembrane potential with the voltage for 50% (V0.5 = −30 mV) activation marked with a purple dot; V0.5 is shown in Fig. 2 A as a purple dashed line. For membrane potentials negative to V0.5, the activation gate starts to close, whereas for potentials positive to V0.5, the activation gate is mostly open. The activation-variable (ua3; Fig. 2 C) rate dependence can be understood based on the AP time course (Fig. 2 A) and ua’s voltage dependence (Fig. 2 E). At slower stimulation frequencies like a cycle length of 750 ms (blue curve in Fig. 2 A), the plateau potential is positive to V0.5 (dashed purple line in Fig. 2 A) for the duration of phase 2 of the AP, keeping ua largely open. It is only at the end of the AP plateau that the membrane potential falls below V0.5, leading to appreciable decreases in ua and return of the activation gate open probability toward 0 (ua3 = 0, closed, Fig. 2 C). In contrast, at faster stimulation frequencies such as at a cycle length of 265 ms (red curve in Fig. 2 A), the plateau potential is negative to V0.5 starting at the end of phase 1 (Fig. 2 A), leading to earlier and more rapid closure of the activation gate (red curve in Fig. 2 C). The activation-gate time constant is in the range of 1–6 ms (Fig. S1 D), which allows the activation gate to closely track changes in membrane potential.
Fig. 2 F shows the fast (ui,f, solid black line) and slow (ui,s, black dashed line) inactivation gating variables as a function of test potential, with the slow inactivation-gating variable V0.5 marked by a teal dot. For membrane potentials positive to V0.5 (teal dot), the slow inactivation gate starts to close whereas for membrane potentials negative to V0.5, the inactivation gates are mostly open. Fig. 2 D shows the inactivation gate open probability (ui,f × ui,s) as a function of time at stimulation cycle lengths of 265 ms (red) and 750 ms (blue). The rate independence of ui,f × ui,s (Fig. 2 D, blue versus red) can be explained from the AP time course (Fig. 2 A) and ui’s voltage dependence (Fig. 2 F). For the slow inactivation gate to close, the membrane potential needs to be positive to V0.5 (−5 mV). For the fast inactivation variable, the voltage dependence is even more positive. However, the AP, both at cycle lengths of 265 and 750 ms, spends negligible time at potentials positive to the V0.5 of either fast or slow inactivation (positive to the blue-dashed line in Fig. 2 E). Moreover, the inactivation time constants are >1 s (Fig. S1 B), at least two orders-of-magnitude longer than the time the membrane spends above V0.5. Hence, because the membrane spends very little time positive to V0.5 and the inactivation gate time constants are slow to close, the inactivation gating variables remain mostly in their open state (>0.93 fractional availability, Fig. 2 D) irrespective of activation frequency.
Rate-dependent effects of IKur block on AP properties and underlying mechanism
When IKur is blocked, the plateau voltage is raised and IKr is enhanced, counteracting the repolarization delays caused by IKur block (8). Consequently, to understand the rate-dependent AP changes caused by IKur block, it is essential to analyze the associated changes in IKr. Fig. 3 A shows the steady-state IKr activation-gate variable in the Courtemanche model as a function of test potential, with V0.1 (−28 mV), V0.5 (−14 mV), V0.75 (−7 mV), and V0.9 (0 mV) marked and transposed as dashed lines onto Fig. 3 B. Fig. 3, B–D, shows the AP, IKur, and IKr simulations obtained at a cycle length of 1000 ms under control conditions (blue) and with 75% IKur block (red). IKur block elevates the plateau potential, bringing the membrane potential positive to IKr’s V0.5 (−14 mV; green dashed line in Fig. 3 B) for the duration of phase 2 of the AP, leading to a 136% increase in IKr, an acceleration in phase-3 repolarization and no net change in overall APD (Fig. 3 B; APD−60 control versus IKur block = 255 vs. 256 ms, respectively). Fig. 3, E–G, shows the AP, IKur, and IKr simulations at a cycle length of 250 ms under control conditions (blue) and with 75% IKur block (red). Because of the change in AP morphology at the short cycle length, IKur block-induced elevation of the plateau fails to keep the plateau potential in the IKr activation range (e.g., V0.5 of −14 mV; green dashed line in Fig. 3 E), hence, there is little IKr recruitment (ratio of total IKr with IKur block versus control = 1.40), leading to APD prolongation (APD−60 under control versus IKur-block conditions of 199 and 209 ms, respectively). Therefore, despite the fact that IKur is smaller at rapid frequencies, the ability of IKur block to prolong APD is enhanced.
IKur block and AF termination
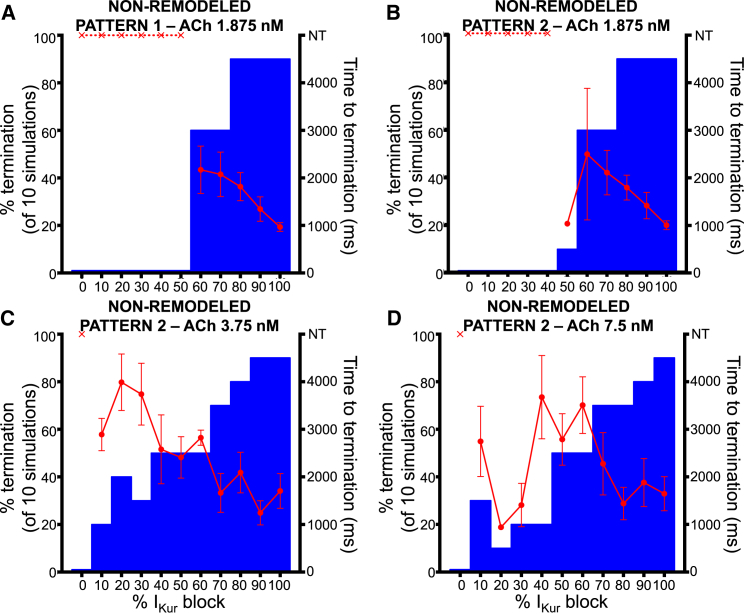
The above analysis suggests that the APD-prolonging effect of IKur blockade is preserved at rapid rates and might result in an ability to suppress AF. We therefore studied the effect of IKur block on simulated two-dimensional cholinergic AF. We generated nine ACh conditions (Fig. S4); reentry was sustained for >5 s across conditions but displayed markedly different dynamics ranging from single spiral wave reentry (Fig. S5) to multiple, short-lived wavelets (Figs. 4, S5, and S6 for representative examples). In line with our single-cell results, there were no significant differences in reentry dynamics, APD−60 distribution, and depolarized fraction between the original and modified IKur inactivation models (Figs. 5, S7, and S8 for representative examples). Using the non-remodeled Courtemanche human action potential model, we found a dose-dependent relationship between AF termination efficacy and percent IKur block (Fig. 6). Reentry termination was relatively infrequent with <50% IKur block, but increased to >90% of simulations at 100% IKur block for all conditions considered. We also found an inverse relationship between average time to termination and percent IKur block (Fig. 6).
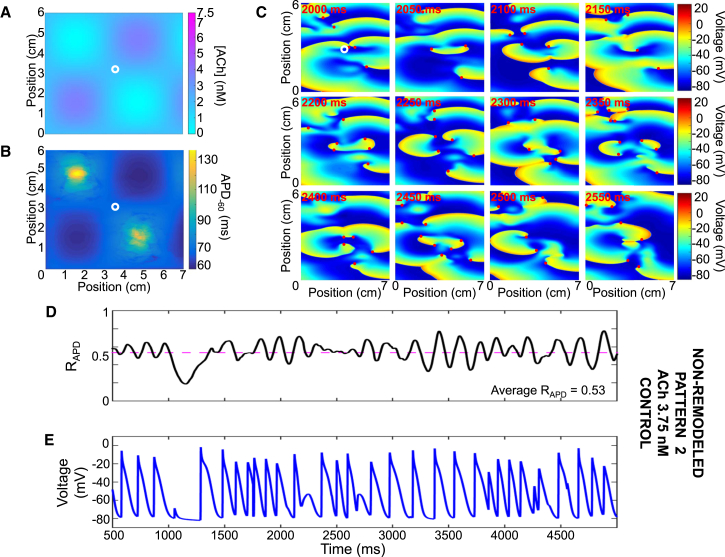
Figure 4.
Representative example of simulated vagotonic AF using pattern #2 with a peak ACh concentration of 3.75 nM and the non-remodeled cardiomyocyte model. (A) Shown here is ACh distribution with peak concentration of 3.75 nM and (B) a corresponding APD−60 distribution. (C) Shown here is transmembrane potential over time at 50-ms intervals; reentry is maintained by multiple short-lived spiral waves. (D) Shown here is the ratio of depolarized cells (ratio of cells with a voltage positive to −60 mV to the total number of cells) and (E) transmembrane potential over time for the cardiomyocyte marked with a white circle in (A) and (B).
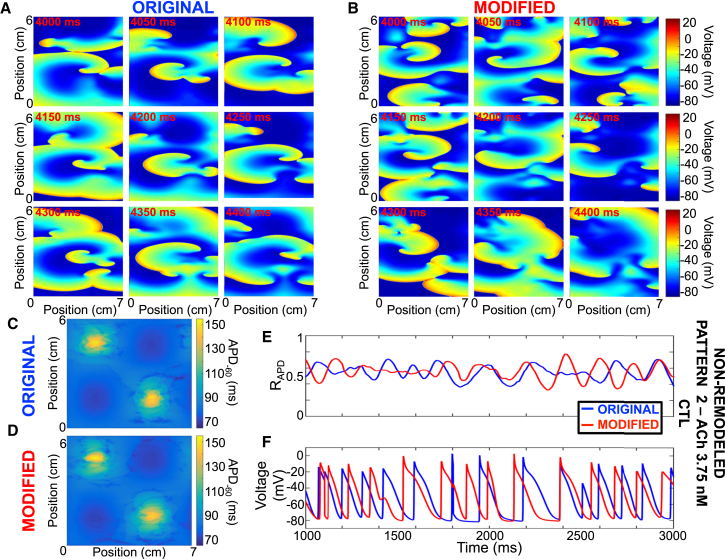
Figure 5.
Representative example comparing reentry dynamics in the original and modified models. (A and B) Transmembrane potential snapshots over time at 50-ms intervals for the original and modified models are shown. (C and D) APD−60 values for the original and modified models are given. (E and F) Shown here is the ratio of depolarized cells (ratio of cells with a voltage positive to −60 mV to the total number of cells) and transmembrane potential for the original (blue) and modified (red) models. Non-remodeled cardiomyocyte model with ACh pattern #2 with peak concentration of 3.75 nM is given.
Figure 6.
Dose-response (bar-graphs) and average time to termination (red data) by IKur block using the non-remodeled cardiomyocyte model for (A) ACh pattern #1 with peak ACh concentration of 1.875 nM. (B–D) Shown here are the ACh pattern #2 and peak ACh concentrations of 1.875, 3.75, and 7.5 nM, respectively.
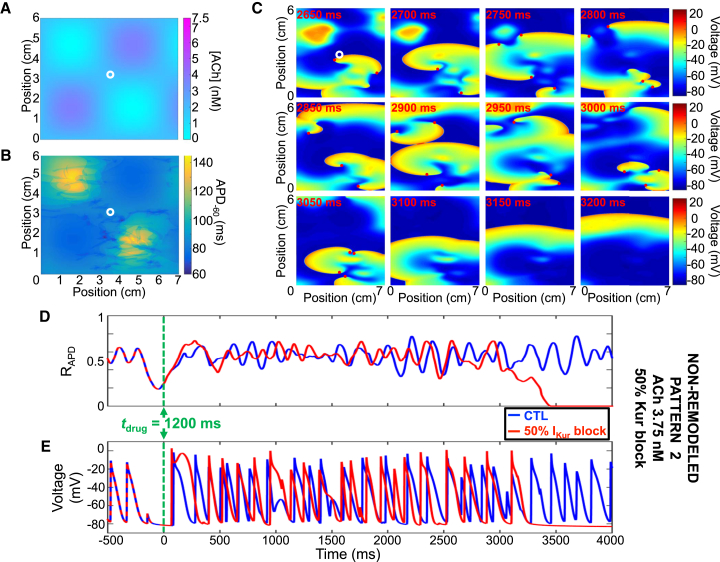
Fig. 7 shows a representative example of successful AF termination by 50% IKur block with ACh pattern #2 and a peak concentration of 3.75 nM (Fig. 7 A). The areas of largest ACh concentration had the shortest APs (Fig. 7 B). IKur block prolonged refractoriness, increasing the excursion of the phase singularities, favoring wave-front collision, annihilation, and reentry termination (Fig. 7, C–E). Fig. S9 shows the dynamics with the same ACh pattern/concentration but with 100% IKur block; the increased refractoriness is even more pronounced and termination more rapid. Consistent with a significant role of IKur in AP repolarization, the mean APD−60 increased from 85 ms under control conditions to 97 and 126 ms at 50 and 100% IKur block, respectively.
Figure 7.
Representative example of reentry termination by 50% IKur block using ACh pattern #2 with peak ACh concentration of 3.75 nM and the non-remodeled cardiomyocyte model. (A)Shown here is the ACh distribution with peak concentration of 3.75 nM and (B) its corresponding APD−60 distribution. (C) Transmembrane potential snapshots over time at 50 ms intervals are given; 50% IKur block was introduced at tdrug = 1200 ms. (D) Shown here is the ratio of depolarized cells (ratio of cells with a voltage positive to −60 mV to the total number of cells) and (E) transmembrane potential over time for the cardiomyocyte marked with a white circle in (A) and (B) for control (blue) and 50% IKur block (red).
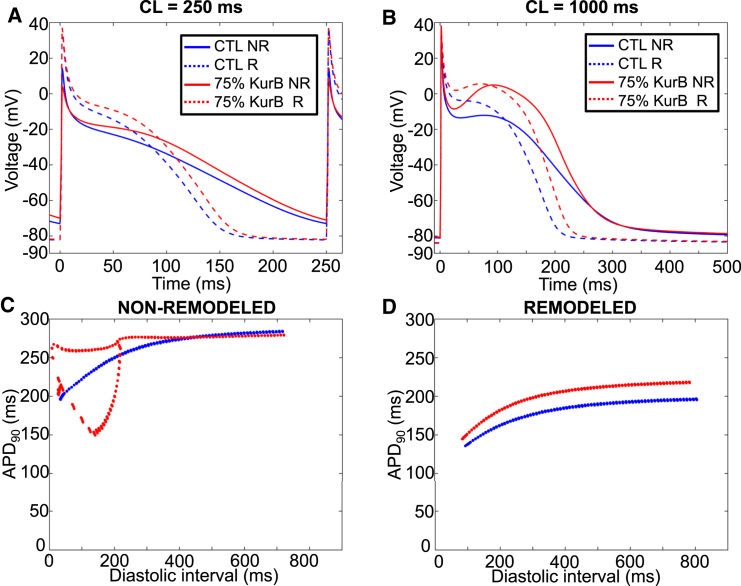
We then sought to investigate whether IKur block could successfully terminate two-dimensional AF under ionically remodeled conditions, such as in chronic AF. As previously reported (7), AF-remodeling conditions stabilize reentry dynamics (Figs. S10–S12) and ACh is no longer needed to maintain AF. Overall, the efficacy of IKur block in terminating AF in remodeled atria was very low, with no termination observed below 80% block and 10% termination at 90 and 100% block in the absence of ACh and across the nine ACh conditions (see Fig. S13 for a representative example). At the cellular level, remodeling stabilized reentry 1) by significantly shortening the APD90 (APD90 for non-remodeled versus remodeled was 283 vs. 195 ms, respectively at CL 1000 ms and 204 vs. 140 ms, respectively at CL 250 ms) and 2) by hyperpolarizing the resting membrane potential (RMP) as compared to the non-remodeled cardiomyocyte (Fig. 8, A and B, dashed versus solid) across a wide range of diastolic intervals. The IKur block-induced APD90 prolongation was preserved under remodeled conditions (Fig. 8 C versus Fig. 8 D, red versus blue; ΔAPD90 at CL = 250 ms non-remodeled versus remodeled was 11 vs. 10 ms, respectively). However, because the remodeled AP is of much shorter duration and is hyperpolarized, the diastolic interval is long enough for the AP to return to the RMP before the next activation, even at rapid stimulation frequencies (Fig. 8 A, blue versus red dashed). Conversely, for the non-remodeled cardiomyocyte, even a small prolongation in APD encroaches on repolarization at short CLs (Fig. 8 A, blue versus red solid), and IKur block causes APD alternans behavior at short CLs (Fig. 8 C). Hence, the differential efficacy of IKur blockade for AF termination in remodeled versus non-remodeled atria appears to be related to the remodeling-induced APD-abbreviation and hyperpolarization more than from IKur downregulation, because the APD prolongation caused by IKur block is preserved in remodeled cardiomyocytes.
Figure 8.
IKur blocking effects in remodeled cardiomyocytes. (A) Shown here is a single cell AP at a stimulation CL of 250 ms for a non-remodeled (NR; solid) and remodeled (R; dashed) cardiomyocyte without drug (blue) and with 75% IKur block (red); (B) same as in (A), but at CL of 1000 ms. (C) Shown here is an AP at 90% repolarization (APD90) as a function of diastolic interval without drug (blue) and with 75% IKur block (red) for a non-remodeled cardiomyocyte; (D) same as in (C), but for a remodeled cardiomyocyte.
Discussion
In this study, we have developed an updated formulation of IKur inactivation kinetics that reproduces experimentally observed IKur inactivation properties. Using the model, we have shown that, under physiological stimulation conditions, IKur dynamics are mainly determined by its activation kinetics with relatively minor contribution from channel inactivation. Hence, 1) contrary to intuition, IKur inactivation does not accumulate at rapid stimulation frequencies; and 2) despite a rate-dependent decrease in absolute IKur magnitude, the relative contribution of IKur to AP repolarization increases with increased activation frequency. We also found that IKur block terminates simulated cholinergic AF in a dose-dependent fashion in non-remodeled atrial tissue but is ineffective in the presence of simulated atrial remodeling. Because IKur is expressed in the human atrium but not ventricle (9), it is an interesting potential candidate for atrial-selective anti-AF therapy. Therefore, the demonstration that despite its potential for complete inactivation, IKur maintains a significant contribution to repolarization dynamics and arrhythmia maintenance during AF, is potentially important.
IKur inactivation
The initial reports characterizing IKur inactivation used relatively short test pulses, typically <5 s long, and therefore failed to observe the full scale of IKur inactivation (1, 10, 11, 12, 13, 14). Using 50-s test pulses, Feng et al. (2) demonstrated that human atrial IKur inactivates fully, with a biexponential time-course characterized by rapid and slow time constants of 1.0 and 6.8 s, respectively. Moreover, IKur inactivation was found to be highly time-, voltage-, and frequency-dependent. These observations raised the possibility that channel inactivation could accumulate at rapid activation frequencies, significantly decreasing IKur’s contribution to AP dynamics. If IKur inactivated completely at rapid activation frequencies, such as during AF, then IKur blockers would be predicted to have minimal antiarrhythmic effects. Paradoxically, and contrary to this intuitive expectation, recent work with the highly selective IKur blocker XEN-D0103 found that IKur block prolongs the atrial APD and effective refractory period preferentially at rapid activation frequencies (3).
Novel elements relative to prior in silico work
To our knowledge, none of the commonly used human atrial in silico models correctly reproduces the experimentally observed IKur inactivation kinetics. In this investigation, we first analyzed experimental recordings of IKur inactivation to produce an accurate in silico representation. We then studied IKur inactivation dynamics and rate-dependence with the updated model that accounted realistically for IKur inactivation (2) by introducing a set of fast and slow inactivation gating variables (ui,f and ui,s) and corresponding time constants (τui,f and τui,s). The modified model accurately reproduced IKur time-, voltage-, and frequency-dependent inactivation across a wide range of physiological test potentials. Of note, the inactivation-gate time constants are three orders-of-magnitude slower than the activation-gate time constant (second versus millisecond). After including the updated IKur representation in the Courtemanche AP-model, we compared AP parameters as a function of stimulation cycle length and found no significant difference between the original and modified model results despite markedly different inactivation kinetics. We then applied our model to gain insight into the role of IKur activation and inactivation on IKur and its rate-dependent role in repolarization and arrhythmia maintenance.
Mechanism of IKur rate dependence
Using the modified model, we showed that the activation gate open probability is highly rate-dependent (because of rate-induced AP morphology changes) and parallels IKur rate dependence, whereas the inactivation gate open probability is effectively rate independent because the cell spends little time at voltages associated with significant inactivation-gate closure. Hence, IKur rate-dependence is due to its activation gating-variable dynamics and rate-dependent changes in AP morphology, with no contribution from inactivation.
Rate-dependent effect of IKur block on AP repolarization
We next sought to understand the mechanism underlying the positive rate-dependent effect of IKur block on the APD, as reported by Ford et al. (3). At slow stimulation frequencies, IKur block significantly elevated the AP plateau potential compared to control (no block), keeping the membrane at potentials for which IKr’s activation gate opens for the duration of phase 2 of the AP, leading to recruitment of IKr and compensating for IKur blockade with minimal effect on the APD. At rapid stimulation frequencies, the membrane potential is negative to IKr’s half-activation potential leading to little IKr recruitment, limited compensation by IKr, and IKur block-induced APD prolongation at rapid frequencies. Hence, although IKur’s absolute magnitude is less at rapid activation frequencies, the relative contribution of IKur to AP repolarization makes IKur block a potentially interesting atrial-selective anti-AF strategy.
The mechanisms of reverse rate-dependency (RRD) of drug-induced APD changes have been a matter of substantial debate. Arguing from first principles, Zaza (15) proposed that RRD is an intrinsic property of cardiac cells and that the same change in total transmembrane current will prolong long APs more than short ones. These predictions were supported by experimental work in which APD changes were shown to be proportional to the initial APD using clinically available drugs like the class-I agent lidocaine and class-III agent dofetilide, as well as by applying inward or outward current pulses (16). The same group suggested that channel blockers may, at best, attenuate this intrinsic RRD but that forward rate-dependency (FRD) would be “difficult to attain” (16). In an elegant study using a series of ventricular cardiomyocyte models, Cummins et al. (17) challenged this notion by suggesting that RRD can be overcome if rate-dependent AP morphological changes are large enough. In other words, the complex nonlinear rate-dependent changes in current dynamics may be such that RRD could be offset, making FRD possible. For example, they showed that increasing ICa,L (gCa,L) leads to FRD changes by elevating the AP plateau potential, leading to differential IKs activation at slow activation frequencies (17). Because IKur is not expressed in ventricular cardiomyocytes, the possible FRD properties of IKur block could not be evaluated. Our findings in atrial cardiomyocytes are, however, qualitatively in line with these observations, because we found that IKur block moves the AP plateau potential to more positive voltages, leading to differential IKr activation at slow versus rapid pacing rates and FRD APD-prolongation. Hence, FRD APD-prolongation, a highly desirable antiarrhythmic property, may be an attainable goal.
Relevance for antiarrhythmic drug development
We also found that IKur block terminates simulated cholinergic AF in a dose-dependent manner in nonionically remodeled atrial tissue by prolonging refractoriness, increasing the reentry wavelength, favoring rotor collision and annihilation. These findings are consistent with prior experimental work using relatively IKur-specific blockers in goats, rats, pigs, humans, and in silico systems (18, 19, 20, 21, 22). Electrical remodeling shortened the APD and hyperpolarized the RMP, leading to very stable reentry as described in Pandit et al. (7). The ability of IKur block to terminate simulated AF was greatly attenuated by remodeling, because the block-induced APD-prolongation was insufficient to counteract the strong effects of remodeling. The APD-prolonging effect of IKur block was not per se affected by channel downregulation. These observations are consistent with the report by Ford et al. (3), in which APD90 prolongation induced by the IKur-selective blocker XEN-D0103 was maintained in chronic-AF human atrial cardiomyocytes. Scholz et al. (22) found that IKur block was effective at terminating simulated AF, even in remodeled atria. However, they used slightly different remodeling parameters, included gap junction remodeling, and did not incorporate IKur downregulation. The differential effectiveness of IKur block in non-remodeled versus remodeled atria is consistent with a prior randomized phase-3 clinical trial in which vernakalant, a mixed INa/IKur blocker, was much less effective at restoring sinus rhythm in patients with long-lasting AF (4.0%) compared to recent-onset AF (51.7%) (23). Of note, recent experimental work demonstrated persistent antiarrhythmic efficacy of vernakalant in goats with remodeled atria owing to unaltered effects on Na+-dependent parameters (conduction velocity and postrepolarization refractoriness) (24). Our findings suggest that the clinical efficacy of pure IKur block may be limited to recent-onset AF, in which remodeling has not taken place.
For the purposes of this study, IKur block was simulated as a fixed reduction in maximal conductance (gKur). However, there is in silico and experimental evidence that the electrophysiological and antiarrhythmic effects of IKur blockade are modulated by the time- and voltage-dependent kinetics of block. Using a family of IKur-selective diphenyl phosphine oxide compounds, Lagrutta et al. (25) were able to show that IKur blocking potency and frequency dependence were functions of blocking kinetics, with open state blockers being the most effective IKur antagonists. Several other molecules, including vernakalant and experimental compounds like zatebradine, loratadine, and bisindolylmaleimide, have also been shown to block IKur preferentially in the open state (19, 26, 27, 28). Mathematical simulations support these findings and suggest that IKur blockers with rapid binding or slow unbinding kinetics have the strongest antiarrhythmic effects (22, 29). Further work is needed to analyze the effects (if any) on state-dependent drug block, of adding realistic IKur inactivation kinetics.
Consistent with this study, XEN-D0101, a selective IKur blocker, prolonged the atrial effective refractory period (AERP) and decreased AF vulnerability in a dose- and rate-dependent manner in atrial tachycardia-induced remodeled canine atria (30, 31). In human cardiomyocytes, XEN-D0101 prolonged AERP in AF-remodeled but not in non-remodeled tissue (32). However, experiments were conducted using a stimulation frequency of 1 Hz at which we predict no effect on APD and AERP based on this study; rapid stimulation rates at which IKur-block induced prolongation of APD/atrial refractoriness would be expected were not reported. In coronary-perfused canine atria, 4-aminopyridine (a moderately selective IKur blocker) was found to shorten APD and to increase the propensity for AF, displaying only mildly antiarrhythmic effects in remodeled atrial tissue (33). Again, the protocol employed a stimulation cycle length of 500 ms at which IKur block would not be expected to increase the APD or AERP. Furthermore, the mechanism of IKur in dog atrium is different from that in humans, and the magnitude is often very small (34). MK-0448 failed to prolong AERP in healthy human subjects using relatively slow stimulation cycle lengths of 400 and 600 ms at which IKr recruitment balances IKur block (35). IKur block rate-dependence, as described in this study, along with species- and remodeling-related differences, likely account for these conflicting results. Finally, genetic studies reported both loss- and gain-of-function variants associated with the development of lone AF (36, 37, 38, 39). Whether and how these observations in rare genetically based forms of AF are applicable, remains to be seen more broadly.
Na+ channel blockers (NCBs) are moderately effective antiarrhythmic drugs commonly used to control AF (40). In 2015, it was reported that IKr block increases the anti-AF effects of an optimized NCB by delaying repolarization at rapid rates (41). However, IKr blockers have reverse-use-dependent effects on APD, such that the APD-prolonging effect of IKr block is maximal at the slow rates of normal sinus rhythm (producing a serious risk of excess repolarization delay and ventricular proarrhythmia) and decreases markedly at rapid rates like those of AF. Given the positive rate-dependence of the APD-prolonging effect of IKur block, the addition of an IKur blocker to an optimized NCB would be expected to potentiate the NCB’s anti-AF efficacy preferentially at rapid rates such as during AF. We have already shown evidence for this principle in a canine computational model (41). The work presented here provides tools and further rationale for testing this concept in human models.
Study limitations
First, this study was performed in silico with extensive use of prior primary experimental patch-clamp data (2). The model predictions regarding the rate-dependent effects of IKur block on AP properties need to be tested prospectively in human tissue. Second, the model does not consider IKur modulation by adrenergic (42) or by vagal (30) tone, nor by temperature dependence (2). However, the model was based on data obtained at normal body temperature and should therefore be relevant to normal clinical conditions. Finally, as discussed above, we simulated IKur block with a fixed reduction in maximal conductance, whereas IKur block by antiarrhythmic drugs have been shown to depend on blocking kinetics and show state-dependent properties (22, 29, 43). Further computational analyses considering the state-dependent actions of specific IKur blockers might therefore be of interest.
Our simulations in remodeled atria have one major limitation. To compare remodeled results with those in non-remodeled conditions, we used the same IKACh model and distributions. However, IKACh is greatly reduced in remodeled atria (44). There are no realistic IKACh models for remodeled atria. Future work is needed to create such models and use them to obtain a more accurate picture of IKur-blocking effects under these conditions.
Conclusions
An updated in silico model that accounts for experimentally observed IKur inactivation kinetics shows that, contrary to possible intuitive inferences based on the potentially complete inactivation shown by IKur, IKur inactivation is in fact negligible under physiologically relevant conditions. On the contrary, the main determinant of IKur rate dependence is the response of its activation dynamics to frequency-dependent changes in AP-morphology. However, despite a smaller absolute IKur magnitude at rapid rates, the relative contribution of IKur to AP repolarization increases because of decreases in offsetting currents, particularly IKr. These positive rate-dependent effects allow IKur block to terminate AF and position it to have potentially valuable antiarrhythmic properties. At the same time, our results also suggest that the efficacy of IKur block for AF termination might be greatly attenuated in the ionically remodeled atrium that develops after several days or more of sustained AF (8).
Author Contributions
M.A. designed the study with the assistance of P.C. and S.N., performed all the simulations, analyzed the data and composed the manuscript. J.F. performed the original experimental work, provided all the original experimental data for analysis, and helped with the interpretation of the experimental results. E.V. provided the code for the AF simulation and assisted in its execution/analysis. P.C. supervised the modeling work and provided comments to improve the manuscript. S.N. supervised all aspects of the work, contributed to the original study concept and design, and helped with planning and completion of the manuscript.
Acknowledgments
We thank Jennifer Bacchi for expert secretarial assistance with the manuscript.
We acknowledge support through the Canadian Institutes of Health Research and Quebec Heart Foundation (S.N.), the Natural Sciences and Engineering Research Council (P.C.), and the Agence National de Recherche de France through the Investissements d’Avenir Program No. ANR-10-IAHU-04 (E.V.). Computations were made on the supercomputer Briare from Université de Montréal, managed by Calcul Québec and Compute Canada. The operation of this supercomputer is funded by the Canada Foundation for Innovation (CFI), the ministère de l’Économie, de la science et de l'innovation du Québec (MESI), and the Fonds de recherche du Québec - Nature et technologies (FRQ-NT).
Editor: Eric Sobie.
Footnotes
Philippe Comtois and Stanley Nattel contributed equally to this work.
Thirteen figures are available at http://www.biophysj.org/biophysj/supplemental/S0006-3495(17)30341-7.
Supporting Material
References
- 1.Wang Z., Fermini B., Nattel S. Sustained depolarization-induced outward current in human atrial myocytes. Evidence for a novel delayed rectifier K+ current similar to Kv1.5 cloned channel currents. Circ. Res. 1993;73:1061–1076. doi: 10.1161/01.res.73.6.1061. [DOI] [PubMed] [Google Scholar]
- 2.Feng J., Xu D., Nattel S. Ultrarapid delayed rectifier current inactivation in human atrial myocytes: properties and consequences. Am. J. Physiol. 1998;275:H1717–H1725. doi: 10.1152/ajpheart.1998.275.5.H1717. [DOI] [PubMed] [Google Scholar]
- 3.Ford J., Milnes J., Ravens U. The positive frequency-dependent electrophysiological effects of the IKur inhibitor XEN-D0103 are desirable for the treatment of atrial fibrillation. Heart Rhythm. 2016;13:555–564. doi: 10.1016/j.hrthm.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Courtemanche M., Ramirez R.J., Nattel S. Ionic mechanisms underlying human atrial action potential properties: insights from a mathematical model. Am. J. Physiol. 1998;275:H301–H321. doi: 10.1152/ajpheart.1998.275.1.H301. [DOI] [PubMed] [Google Scholar]
- 5.Kneller J., Zou R., Nattel S. Cholinergic atrial fibrillation in a computer model of a two-dimensional sheet of canine atrial cells with realistic ionic properties. Circ. Res. 2002;90:E73–E87. doi: 10.1161/01.res.0000019783.88094.ba. [DOI] [PubMed] [Google Scholar]
- 6.Vigmond E.J., Hughes M., Leon L.J. Computational tools for modeling electrical activity in cardiac tissue. J. Electrocardiol. 2003;36:69–74. doi: 10.1016/j.jelectrocard.2003.09.017. [DOI] [PubMed] [Google Scholar]
- 7.Pandit S.V., Berenfeld O., Jalife J. Ionic determinants of functional reentry in a 2-D model of human atrial cells during simulated chronic atrial fibrillation. Biophys. J. 2005;88:3806–3821. doi: 10.1529/biophysj.105.060459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Courtemanche M., Ramirez R.J., Nattel S. Ionic targets for drug therapy and atrial fibrillation-induced electrical remodeling: insights from a mathematical model. Cardiovasc. Res. 1999;42:477–489. doi: 10.1016/s0008-6363(99)00034-6. [DOI] [PubMed] [Google Scholar]
- 9.Feng J., Wible B., Nattel S. Antisense oligodeoxynucleotides directed against Kv1.5 mRNA specifically inhibit ultrarapid delayed rectifier K+ current in cultured adult human atrial myocytes. Circ. Res. 1997;80:572–579. doi: 10.1161/01.res.80.4.572. [DOI] [PubMed] [Google Scholar]
- 10.Philipson L.H., Hice R.E., Steiner D.F. Sequence and functional expression in Xenopus oocytes of a human insulinoma and islet potassium channel. Proc. Natl. Acad. Sci. USA. 1991;88:53–57. doi: 10.1073/pnas.88.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Snyders D.J., Tamkun M.M., Bennett P.B. A rapidly activating and slowly inactivating potassium channel cloned from human heart. Functional analysis after stable mammalian cell culture expression. J. Gen. Physiol. 1993;101:513–543. doi: 10.1085/jgp.101.4.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Firek L., Giles W.R. Outward currents underlying repolarization in human atrial myocytes. Cardiovasc. Res. 1995;30:31–38. [PubMed] [Google Scholar]
- 13.Amos G.J., Wettwer E., Ravens U. Differences between outward currents of human atrial and subepicardial ventricular myocytes. J. Physiol. 1996;491:31–50. doi: 10.1113/jphysiol.1996.sp021194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koidl B., Flaschberger P., Rigler B. Effects of the class III antiarrhythmic drug ambasilide on outward currents in human atrial myocytes. Naunyn Schmiedebergs Arch. Pharmacol. 1996;353:226–232. doi: 10.1007/BF00168761. [DOI] [PubMed] [Google Scholar]
- 15.Zaza A. Control of the cardiac action potential: the role of repolarization dynamics. J. Mol. Cell. Cardiol. 2010;48:106–111. doi: 10.1016/j.yjmcc.2009.07.027. [DOI] [PubMed] [Google Scholar]
- 16.Bányász T., Horváth B., Nánási P.P. Reverse rate dependency is an intrinsic property of canine cardiac preparations. Cardiovasc. Res. 2009;84:237–244. doi: 10.1093/cvr/cvp213. [DOI] [PubMed] [Google Scholar]
- 17.Cummins M.A., Dalal P.J., Sobie E.A. Comprehensive analyses of ventricular myocyte models identify targets exhibiting favorable rate dependence. PLoS Comput. Biol. 2014;10:e1003543. doi: 10.1371/journal.pcbi.1003543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Knobloch K., Brendel J., Wirth K.J. Electrophysiological and antiarrhythmic effects of the novel IKur channel blockers, S9947 and S20951, on left vs. right pig atrium in vivo in comparison with the IKr blockers dofetilide, azimilide, d,l-sotalol and ibutilide. Naunyn-Schiedeberg’s Arch. Pharmacol. 2002;366:482–487. doi: 10.1007/s00210-002-0599-x. [DOI] [PubMed] [Google Scholar]
- 19.Blaauw Y., Gögelein H., Allessie M.A. “Early” class III drugs for the treatment of atrial fibrillation: efficacy and atrial selectivity of AVE0118 in remodeled atria of the goat. Circulation. 2004;110:1717–1724. doi: 10.1161/01.CIR.0000143050.22291.2E. [DOI] [PubMed] [Google Scholar]
- 20.Fedida D., Orth P.M.R., Beatch G.N. The mechanism of atrial antiarrhythmic action of RSD1235. J. Cardiovasc. Electrophysiol. 2005;16:1227–1238. doi: 10.1111/j.1540-8167.2005.50028.x. [DOI] [PubMed] [Google Scholar]
- 21.Dorian P., Pinter A., Beatch G.N. The effect of vernakalant (RSD1235), an investigational antiarrhythmic agent, on atrial electrophysiology in humans. J. Cardiovasc. Pharmacol. 2007;50:35–40. doi: 10.1097/FJC.0b013e3180547553. [DOI] [PubMed] [Google Scholar]
- 22.Scholz E.P., Carrillo-Bustamante P., Seemann G. Rotor termination is critically dependent on kinetic properties of IKur inhibitors in an in silico model of chronic atrial fibrillation. PLoS One. 2013;8:e83179. doi: 10.1371/journal.pone.0083179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roy D., Pratt C.M., Camm A.J., Atrial Arrhythmia Conversion Trial Investigators Vernakalant hydrochloride for rapid conversion of atrial fibrillation: a phase 3, randomized, placebo-controlled trial. Circulation. 2008;117:1518–1525. doi: 10.1161/CIRCULATIONAHA.107.723866. [DOI] [PubMed] [Google Scholar]
- 24.van Hunnik A., Lau D.H., Schotten U. Antiarrhythmic effect of vernakalant in electrically remodeled goat atria is caused by slowing of conduction and prolongation of postrepolarization refractoriness. Heart Rhythm. 2016;13:964–972. doi: 10.1016/j.hrthm.2015.12.009. [DOI] [PubMed] [Google Scholar]
- 25.Lagrutta A., Wang J., Salata J.J. Novel, potent inhibitors of human Kv1.5 K+ channels and ultrarapidly activating delayed rectifier potassium current. J. Pharmacol. Exp. Ther. 2006;317:1054–1063. doi: 10.1124/jpet.106.101162. [DOI] [PubMed] [Google Scholar]
- 26.Valenzuela C., Delpón E., Snyders D.J. Class III antiarrhythmic effects of zatebradine. Time-, state-, use-, and voltage-dependent block of hKv1.5 channels. Circulation. 1996;94:562–570. doi: 10.1161/01.cir.94.3.562. [DOI] [PubMed] [Google Scholar]
- 27.Delpón E., Valenzuela C., Tamargo J. Block of human cardiac Kv1.5 channels by loratadine: voltage-, time- and use-dependent block at concentrations above therapeutic levels. Cardiovasc. Res. 1997;35:341–350. doi: 10.1016/s0008-6363(97)00121-1. [DOI] [PubMed] [Google Scholar]
- 28.Choi B.H., Choi J.-S., Kim M.-S. Direct block by bisindolylmaleimide of rat Kv1.5 expressed in Chinese hamster ovary cells. J. Pharmacol. Exp. Ther. 2000;293:634–640. [PubMed] [Google Scholar]
- 29.Tsujimae K., Suzuki S., Kurachi Y. Frequency-dependent effects of various IKr blockers on cardiac action potential duration in a human atrial model. Am. J. Physiol. Heart Circ. Physiol. 2007;293:H660–H669. doi: 10.1152/ajpheart.01083.2006. [DOI] [PubMed] [Google Scholar]
- 30.Rivard L., Shiroshita-Takeshita A., Nattel S. Electrophysiological and atrial antiarrhythmic effects of a novel IKur/Kv1.5 blocker in dogs. Heart Rhythm. 2005;2:S180. [Google Scholar]
- 31.Shiroshita-Takeshita A., Maltais C., Nattel S. Electrophysiological and atrial antiarrhythmic effects of a novel IKur/Kv1.5 blocker in dogs with atrial tachycardia remodeling. Heart Rhythm. 2006;3:S183. [Google Scholar]
- 32.Ford J., Milnes J., Ravens U. Human electrophysiological and pharmacological properties of XEN-D0101: a novel atrial-selective Kv1.5/IKur inhibitor. J. Cardiovasc. Pharmacol. 2013;61:408–415. doi: 10.1097/FJC.0b013e31828780eb. [DOI] [PubMed] [Google Scholar]
- 33.Burashnikov A., Antzelevitch C. Can inhibition of IKur promote atrial fibrillation? Heart Rhythm. 2008;5:1304–1309. doi: 10.1016/j.hrthm.2008.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yue L., Wang Z., Nattel S. Molecular evidence for a role of Shaw (Kv3) potassium channel subunits in potassium currents of dog atrium. J. Physiol. 2000;527:467–478. doi: 10.1111/j.1469-7793.2000.00467.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pavri B.B., Greenberg H.E., Bloomfield D. MK-0448, a specific Kv1.5 inhibitor: safety, pharmacokinetics, and pharmacodynamic electrophysiology in experimental animal models and humans. Circ. Arrhythm. Electrophysiol. 2012;5:1193–1201. doi: 10.1161/CIRCEP.111.969782. [DOI] [PubMed] [Google Scholar]
- 36.Olson T.M., Alekseev A.E., Terzic A. Kv1.5 channelopathy due to KCNA5 loss-of-function mutation causes human atrial fibrillation. Hum. Mol. Genet. 2006;15:2185–2191. doi: 10.1093/hmg/ddl143. [DOI] [PubMed] [Google Scholar]
- 37.Yang T., Yang P., Darbar D. Novel KCNA5 mutation implicates tyrosine kinase signaling in human atrial fibrillation. Heart Rhythm. 2010;7:1246–1252. doi: 10.1016/j.hrthm.2010.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Christophersen I.E., Olesen M.S., Schmitt N. Genetic variation in KCNA5: impact on the atrial-specific potassium current IKur in patients with lone atrial fibrillation. Eur. Heart J. 2013;34:1517–1525. doi: 10.1093/eurheartj/ehs442. [DOI] [PubMed] [Google Scholar]
- 39.Hayashi K., Konno T., Yamagishi M. Functional characterization of rare variants implicated in susceptibility to lone atrial fibrillation. Circ. Arrhythm. Electrophysiol. 2015;8:1095–1104. doi: 10.1161/CIRCEP.114.002519. [DOI] [PubMed] [Google Scholar]
- 40.Aguilar M., Nattel S. The past, present, and potential future of sodium channel block as an atrial fibrillation suppressing strategy. J. Cardiovasc. Pharmacol. 2015;66:432–440. doi: 10.1097/FJC.0000000000000271. [DOI] [PubMed] [Google Scholar]
- 41.Aguilar M., Xiong F., Nattel S. Potassium channel blockade enhances atrial fibrillation-selective antiarrhythmic effects of optimized state-dependent sodium channel blockade. Circulation. 2015;132:2203–2211. doi: 10.1161/CIRCULATIONAHA.115.018016. [DOI] [PubMed] [Google Scholar]
- 42.Li G.R., Feng J., Nattel S. Adrenergic modulation of ultrarapid delayed rectifier K+ current in human atrial myocytes. Circ. Res. 1996;78:903–915. doi: 10.1161/01.res.78.5.903. [DOI] [PubMed] [Google Scholar]
- 43.Almquist J., Wallman M., Jirstrand M. Modeling the effect of Kv1.5 block on the canine action potential. Biophys. J. 2010;99:2726–2736. doi: 10.1016/j.bpj.2010.08.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dobrev D., Graf E., Ravens U. Molecular basis of downregulation of G-protein-coupled inward rectifying K+ current (IK,Ach) in chronic human atrial fibrillation: decrease in GIRK4 mRNA correlates with reduced (IK,Ach) and muscarinic receptor-mediated shortening of action potentials. Circulation. 2001;104:2551–2557. doi: 10.1161/hc4601.099466. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.