Abstract
Polymeric microcapsules with a light-absorbing dye incorporated in their shell can generate vapor microbubbles that can be spatiotemporally controlled by pulsed laser irradiation. These contrast agents of 6–8 μm in diameter can circulate through the vasculature, offering possibilities for ultrasound (molecular) imaging and targeted therapies. Here, we study the impact of such vapor bubbles on human endothelial cells in terms of cell poration and cell viability to establish the imaging and therapeutic windows. Two capsule formulations were used: the first one consisted of a high boiling point oil (hexadecane), whereas the second was loaded with a low boiling point oil (perfluoropentane). Poration probability was already 40% for the smallest bubbles that were formed (<7.5 μm diameter), and reached 100% for the larger bubbles. The hexadecane-loaded capsules also produced bubbles while their shell remained intact. These encapsulated bubbles could therefore be used for noninvasive ultrasound imaging after laser activation without inducing any cell damage. The controlled and localized cell destruction achieved by activation of both capsule formulations may provide an innovative approach for specifically inducing cell death in vivo, e.g., for cancer therapy.
Introduction
Commercially available ultrasound contrast agents are primarily based on the highly echogenic scattering of small stabilized gas spheres called microbubbles (1), for contrast enhancement of the blood (2). Next to contrast enhancement, microbubbles have also been functionalized with targeting moieties that can adhere to specific clinically relevant biomarkers (3, 4), hereby extending the applicability of microbubbles to molecular imaging with ultrasound. Ultrasound contrast agents have also been developed that can be loaded with drugs (5, 6) for improved delivery of genetic material and drug molecules to cells (6, 7, 8, 9) for theranostic applications (10, 11, 12). This improved delivery to cells is enabled by microbubble oscillations that cause increased membrane permeability, termed sonoporation (6, 8), which is considered to be one of the most promising routes for ultrasound-mediated drug delivery (8).
The efficiency of ultrasound contrast agents for drug delivery has been shown in vivo (13, 14, 15), and extensive literature can be found that investigates the mechanisms by which drugs are delivered to cells through the use of microbubbles and ultrasound (16, 17, 18, 19, 20, 21, 22, 23). Although the mechanisms are still not fully understood, these studies clearly showed that microbubble oscillations are key to a locally increased cell membrane permeability resulting in enhanced drug delivery. However, a delicate balance between drug delivery achieved through viable sonoporation and cell death has been shown (20, 21). These studies reported that the therapeutic windows of irreversible sonoporation resulting in cell death and reversible sonoporation resulting in viable cells suitable for drug delivery are associated with oscillation and displacement of microbubbles (20), and with the size of the resulting pore (21).
Oscillation of microbubbles can either result in stable or inertial cavitation, both of which play a role in enhancing sonoporation (24). Next to microbubbles, other types of agents such as phase-change droplets (25), polymer nanoparticles (26), and microparticles (6, 27) can also induce cavitation. In contrast to microbubbles, these agents offer longer shelf life and prolonged stability (28). Unlike microbubbles that are confined to the blood pool, polymeric particles can be made small enough to extravasate into the interstitial tissue (29, 30). These polymeric micro- and nanoparticles also offer an unprecedented versatility; they can, for example, carry a larger drug payload in their hydrophobic core than microbubbles (31), they can stably entrap a multitude of elements such as metallic nanoparticles (32), generate gas by hydrolysis of the shell material (33), or be shaped as a porous matrix to be stable under sustained ultrasound exposure (34). On the other hand, polymeric microparticles have been shown to be less responsive to ultrasound than microbubbles due to their stiffer shell, which makes ultrasound imaging of intact polymeric microcapsules challenging (35, 36).
To investigate the potential for laser activation of liquid particles and to improve their imaging capabilities, previous studies have incorporated light absorbers either in perfluorocarbon droplets (37) as plasmonic nanoparticles or as a dye in the shell of polymeric capsules (38). It was shown that single polymeric capsules can be triggered by a laser, leading to vaporization and the formation of a bubble. The scattering strength of these vapor bubbles is promising for ultrasound imaging applications. Downsizing the polymeric capsules to nanometer sizes would allow them to extravasate into tumor tissue, which lies beyond the endothelial cell layer lining the blood vessels and which may open up possibilities for delivering cytostatics into the tumor (39). Additionally, the use of an oil with a low boiling point (i.e., perfluoropentane) appears as a viable approach to reduce the vaporization threshold of these light-absorbing particles below the FDA-approved laser intensity limit (40). Until now, little is known on the biological effects of such confined and controlled vaporization events in the vicinity of cells and the imaging and therapeutic windows have to be assessed before these agents can be further developed for clinical use.
In this work, we designed an experiment to study the effects of laser-induced vaporization bubbles in vitro, created by two types of light-absorbing polymeric microcapsules. The first type of capsules is loaded with a high boiling point oil (hexadecane) and the second type contains a low boiling point oil (perfluoropentane). By means of ultra-high-speed bright field imaging combined with fluorescence imaging, we studied the effects of these laser-activated polymeric microcapsules on human endothelial cells, and we particularly assessed the resulting effect of the created vapor bubbles on the cells. All measured quantities were therefore considered as a function of the vapor bubble properties, e.g., size and lifetime. For example, we recorded the microsecond timescale dynamics of the vapor bubbles to relate these to both cell poration and cell viability. The uptake of a model drug upon cell poration was quantified dynamically and was related to the vapor bubble size and cell death.
Materials and Methods
Capsule production
Hexadecane-loaded polymethylmethacrylate (PMMA) microcapsules were prepared by an emulsion solvent evaporation technique, using microsieve emulsification (41). Before emulsification, hexadecane (H6703; Sigma-Aldrich, Zwijndrecht, the Netherlands), PMMA (Tg = 103°C, Mw 120,000; Sigma-Aldrich), and Sudan Red 7B (Sigma-Aldrich) dyes were dissolved in dichloromethane (Sigma-Aldrich) to achieve a dye concentration of 4.85% w/w.
Poly(lactic-co-glycolic acid) Resomer RG502 microcapsules (hereafter called “Resomer”) containing perfluoropentane oil (PFP) (Fluoromed, Round Rock, TX) were fabricated in a similar way as the PMMA capsules. Poly(lactic-co-glycolic acid) (Tg = 50°C; Corbion Purac, Amsterdam, the Netherlands) was dissolved in dichloromethane along with PFP and Sudan Red 7B dye, and only with PFP for the control capsules, and was placed in a 20°C bath to ensure full miscibility of the oil in dichloromethane. Ultrapure water containing an emulsifier (polyvinyl alcohol 4% (w/w) or sodium cholate 1.5% (w/w)) maintained at <15°C was used as the continuous phase. The solutions were filtered through a 0.45 μm PTFE filter and emulsified through a microsieve membrane (Nanomi, Oldenzaal, the Netherlands) with uniform pores. The emulsions were then spread in an aqueous solution containing the aforementioned emulsifiers. This was left to stir at room temperature for at least 3 h to evaporate the dichloromethane. The hardened microcapsules were concentrated and washed repeatedly by vacuum filtration and 0.05% w/w Tween 20 solution (VWR, Amsterdam, the Netherlands). Subsequently, the washed suspensions were stored at 4°C. Size distributions and concentrations were determined using a Multisizer 3 Coulter Counter (average of n = 3; Beckman Coulter, Mijdrecht, the Netherlands) equipped with a 100-μm aperture. Both capsule types were monodisperse, but PMMA-hexadecane capsules were smaller (average diameter 6.4 μm) than Resomer-PFP capsules (average diameter 7.5 μm). Undiluted concentrations were 7.3 × 107 PMMA-hexacecane capsules/mL and 6.8 × 107 Resomer-PFP capsules/mL (see Supporting Material for size distributions).
Endothelial cell culture
Human umbilical vein endothelial cells (HUVEC) (C2519A; Lonza, Verviers, Belgium) were cultured in T75 flasks (353136; BD Falcon Fisher Scientific, Breda, the Netherlands) in 10 mL EGM-2 medium (CC-3162; Lonza) in a humidified incubator under standard conditions (37°C and 5% CO2). Cells were harvested (passage number 6) from the flask using trypsin in EDTA (CC-5012; Lonza) and replated on one side of an OptiCell (Thermo Scientific, Nunc, Wiesbaden, Germany) in 10 mL EGM-2 medium. Two days later, the cells reached 100% confluence and were ready to be used in the experimental setup.
Experimental setup
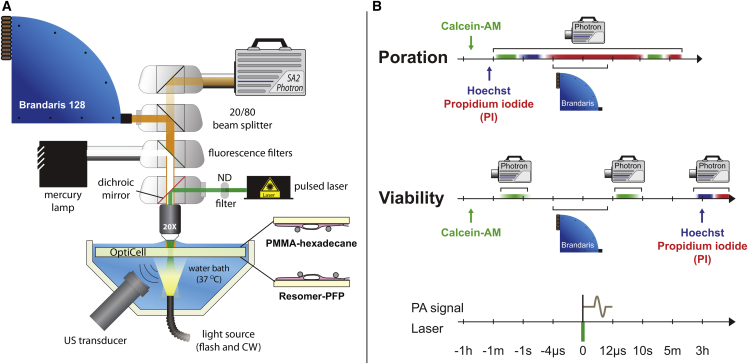
The experimental setup is shown in Fig. 1 A. For visualization of the capsules and the cells, we used a fluorescence microscope (Olympus, Zoeterwoude, the Netherlands) equipped with a 20 water-immersion objective (UMPLFLN 20XW, N.A = 0.5; Olympus). For imaging in bright field, the sample was illuminated from below via a light fiber using a continuous light source (KL2500LED; SCHOTT, Elmsford, NY) and for fluorescence imaging we used a mercury lamp in an epifluorescent setup. In addition, a set of fluorescence filters was used for the detection of propidium iodide (PI) (U-MWG2 filter; Olympus), Hoechst 33342 (U-MWU2 filter; Olympus), and calcein (U-MWIB2; Olympus). For excitation of the polymeric capsules, a pulsed laser (8 ns, 532 nm, EverGreen 150 mJ; Quantel Laser, Newbury, UK) was directed through the microscope on the sample using a dichroic mirror. Different laser energies were used by adjusting the internal settings of the laser, the settings of a polarized attenuator, and a set of neutral density filters. The resulting energy coupled into the microscope was varied from 0.75 to 3.75 mJ for the PMMA-hexadecane capsules and from 0.23 to 2.25 mJ for the Resomer-PFP capsules.
Figure 1.
Setup and timing. (A) Experimental setup comprising a temperature-controlled bath in which the OptiCell containing the cells and the capsules was immersed. An Olympus microscope was used for excitation by the laser, bright field recordings were made using the Brandaris 128 ultra-high-speed camera, and both bright field and fluorescent images were recorded using a Photron SA2 high-speed camera. A 1-MHz focused ultrasound transducer recorded the generated acoustic pressures. (B) Timing scheme for the poration and cell viability experiments. To see this figure in color, go online.
Two cameras were used for imaging: a FASTCAM SA2 high-speed color camera (Photron (Europe), West Wycombe, Buckinghamshire, UK) for bright field and fluorescence imaging, and the Brandaris 128 ultra-high-speed camera (42, 43) for bright field recording of the laser-activated bubble dynamics. For enabling simultaneous recordings of fluorescence and ultra-high-speed bright field imaging, a beamsplitter was used that directed 20% of the light to the high-speed camera and 80% of the light to the Brandaris 128 camera. The high-speed camera recorded at 50 frames per s and the Brandaris 128 ultra-high-speed camera recorded at 10,000,000 frames per s. Sufficient illumination was provided by a Xenon strobe light through the same optical fiber as the continuous light source.
The acoustic signal was received by a focused 1 MHz transducer (C302, 90% bandwidth; Panametrics, Olympus) and was amplified 5× (SR445A; Stanford Research Systems, Sunnyvale, CA). The transducer was mounted in the water bath (37°C) at a 45° angle below the OptiCell. The optical and acoustical foci were aligned using a 0.2-mm metal needle. The acoustic signals were recorded using an oscilloscope (DPO4034; Tektronix, Beaverton, OR) and were automatically saved to a computer using the software MATLAB (The MathWorks, Natick, MA). The main trigger sent by the Brandaris 128 camera was received by a pulse-delay generator (BNC model 575) that in turn controlled the timing of the laser, the flash source, exposure of both cameras, and recording of the acoustic signals.
Poration experiments
The poration experiments were used to study permeabilization of the cell membranes by means of fluorescent stains. For visualization of live cells, we used calcein-AM (C3100MP; Molecular Probes, Life Technologies, Bleiswijk, the Netherlands), a dye that can passively cross the cellular membranes and is enzymatically converted into fluorescent calcein by live cells. We used the efflux of calcein also as a measure of poration (44), in combination with the inflow of the membrane-impermeable model drug PI, that can only enter a cell when the membrane integrity is compromised (45, 46). Before each experiment, calcein-AM was added to the cells in the OptiCell (final concentration 0.4 μg/mL prepared from a 1 μg/mL stock in dimethyl sulfoxide, D5879; Sigma-Aldrich) and was incubated for 40 min (37°C, 5 CO2). Before each experiment, PI (final concentration 25 μg/mL; P-4864; Sigma-Aldrich) and Hoechst 33342 (final concentration 5 μg/mL; H3570; Molecular Probes, Life Technologies) were added to the OptiCell. The Hoechst stain was used to label the nuclei of all cells, allowing for accurate cell counting. For the capsules, ∼1 × 107 PMMA-hexadecane capsules or 2 × 106 Resomer-PFP capsules were added to an OptiCell containing a monolayer of HUVECs. The OptiCell was placed in a 37°C water bath. The PMMA-hexadecane capsules float, so in these experiments the OptiCell was placed with the cells on the top membrane to ensure that the capsules were close to the cells. For the experiments on Resomer-PFP capsules, the OptiCell was placed with the cells on the bottom membrane, because these capsules sink (Fig. 1 A). The timing of the poration experiments is illustrated in Fig. 1 B. A field of view (FOV) with live cells, i.e., presence of calcein signal and absence of PI signal before laser activation, was chosen and the high-speed camera recorded the initial Hoechst and calcein fluorescent signals. Immediately after that, a Brandaris 128 recording was initiated, the capsules were activated by the laser, and their dynamics were captured by the Brandaris 128 camera and PI uptake was simultaneously recorded using the high-speed camera. The fluorescence PI recording lasted for ∼10 s after the Brandaris recordings had finished. 1–2 min after that, another PI fluorescence image set was recorded to assess the prolonged uptake caused by the vapor bubbles that were formed.
Assessment of cell viability
To assess long-term (3 h) cell viability, separate experiments were required in which PI was added 3 h after laser activation of the capsules, to visualize which cells had membranes that were still disrupted. A custom-made grid was put on an OptiCell with a confluent layer of HUVECs to mark the same irradiated spot before and after incubation. Calcein-AM was used for staining live cells and was added 40 min before the experiment to a final concentration of 0.4 μg/mL in the OptiCell. Before starting the experiment, ∼1 × 107 PMMA-hexadecane capsules or 2 × 106 Resomer-PFP capsules were added. Directly after laser irradiation, the Brandaris 128 camera recorded the bubble dynamics in bright field and the high-speed camera recorded the calcein signal before and after activation to assess live cells and poration. After the last recording the OptiCell was incubated for 3 h to study long-term cell viability. After this time had elapsed, PI and Hoechst were added to the OptiCell to a final concentration of 25 and 5 μg/mL, respectively. Fluorescence was recorded again for calcein, Hoechst, and PI at the same activation spot as before. The timing of the viability experiments is shown in Fig. 1 B.
Capsule and laser toxicity
Next to the effect of the created vapor bubbles, the capsule toxicity and the influence of the laser intensity on the cells also had to be accounted for. Cell viability of HUVECs in the presence of PMMA-hexadecane and Resomer-PFP capsules (i.e., capsule toxicity) was assessed by means of PI staining in the viability experiments at locations without laser activation. Cell viability was defined as the ratio of live cells and the total number of cells [(number of Hoechst-stained nuclei minus number of cells showing PI uptake)/number of Hoechst-stained nuclei]. The total cell death count in the viability experiments was corrected for the cell death in the presence of the capsules alone, in principle to correct for cell death due to handling of the cells during the experiments. Next to toxicity of the capsules themselves, interaction with the laser could cause local heating of the cells due to absorption of the laser energy. To assess this effect we used Resomer-PFP capsules with similar composition, but in which no Sudan Red 7B was incorporated in the shell, which prevented the capsules from vaporization upon laser irradiation. These experiments were performed with the highest laser intensities that were applied in our experiments.
Image analysis and signal processing
Data processing was performed entirely with the software MATLAB (The MathWorks, Natick, MA). Image registration had to be performed, because the Brandaris 128 camera had a smaller FOV and was rotated with respect to the high-speed camera. A two-dimensional cross-correlation method was used to register the fluorescence recordings with the corresponding bright field Brandaris recordings. The fluorescence images were contrast-enhanced to facilitate analysis, because the calcein images had limited signal intensity.
Automatic counting of the Hoechst-stained nuclei (live and dead cells) and PI-stained cells (porated or dead cells) was achieved through Gaussian convolution filtering (20 pixels, Gaussian width = 10 pixels) and thresholding (10 levels for an image depth of 8 bits) to filter the noise, and detection by counting the local image maxima (imregionalmax function). To extract the PI uptake curves, images were filtered using singular value decomposition (47), after removal of the median pixel intensity taken from the part of the image that was not exposed to the laser. Finally, the pixel intensity was summed for each frame to produce a poration curve, which was later rescaled to the number of porated cells in the FOV. First, free fitting to an exponential curve was applied. Because the noise was too large to allow reliable fitting of the two independent parameters, for all curves the characteristic time was fixed to be the median of the fitted characteristic times. This allowed a fit to the amplitude in a second step. As an objective measure of the poration speed the initial velocity of the curve was calculated, which is represented by the prefactor (or amplitude) divided by the characteristic time. In most cases, more than one bubble was formed within the FOV; we therefore report the average maximum radius of these bubbles.
The Brandaris recordings were used to extract the radius-time curves of the vapor bubbles, resulting from the laser activation. These radius-time curves were extracted using a MATLAB script developed in-house that was based on contrast compensation, image equalization, two-dimensional convolution filtering, background subtraction, and local thresholding. Pixel sizes were converted into micrometers using recordings of a test target with known resolution (USAF 1951 Resolution Test Targets; Edmund Optics, York, UK). The resulting set of images, radius-time curves, and cell counts were interpreted manually: PI uptake and calcein efflux were attributed to a bubble when this was in close proximity to the affected cell.
Results
Endothelial cell membrane poration by PMMA-hexadecane capsules
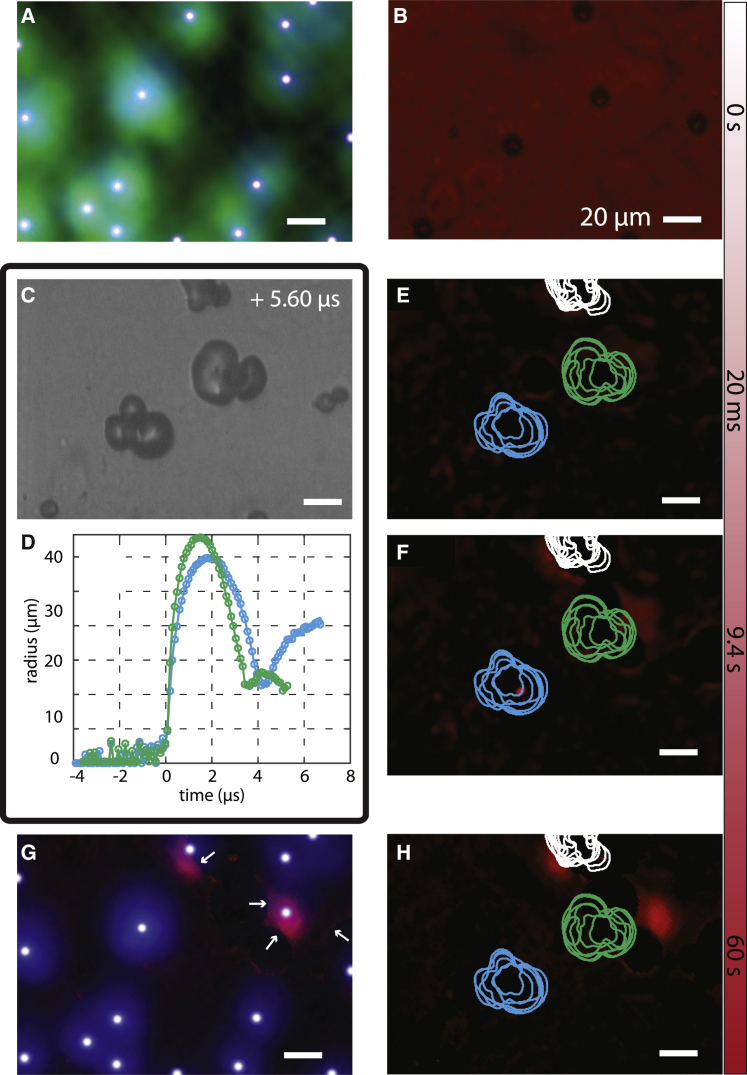
Fig. 2 shows the result of a typical poration experiment using PMMA-hexadecane microcapsules. This figure shows the overlay of calcein and corresponding Hoechst signal of the cells (Fig. 2 A) and the capsules and absence of PI uptake (Fig. 2 B). The capsules just after laser irradiation are shown in Fig. 2 C; Movie S1, and an example of a radius-time curve is shown in Fig. 5 E. The PI uptake curve of the sample, normalized to the number of bubbles in the FOV, is shown in Fig. 2 D, where a clear increase in intracellular PI fluorescence intensity was observed after laser activation. Example frames from this fluorescence recording are shown in Fig. 2, E and F; the time points of these frames correspond to the black dots in Fig. 2 D. Fig. 2 E shows the same image as Fig. 2 B, but now with background subtraction to remove the capsules and focus on the PI signal only. The contours of the vapor bubbles, which were tracked simultaneously, are superimposed on the PI images. The different lines indicate the contour of the bubble in separate frames of the recording. Another PI fluorescence image, taken after ∼1 min, is displayed in Fig. 2 H. The fluorescence intensity in Fig. 2 H clearly increased locally, and four cell nuclei became visible through increased PI fluorescence staining. The four affected cells were observed to be in direct contact with the vapor bubbles. Because the concentration of genetic material to which PI can bind is higher in the nucleus (DNA) than in the cytoplasm (RNA) (48), we only observed a clear signal increase in the nucleus. The overlay of Hoechst fluorescence recordings before and PI recordings after activation (Fig. 2 G) clearly shows poration of three cells that were alive before laser activation. The PI staining of the nucleus shows slight displacement of the cells with respect to the location of the nucleus before the experiment.
Figure 2.
Cell membrane poration PMMA-hexadecane capsules. (A and B) Set of images showing the situation before laser irradiation. The overlay of the calcein (green) and Hoechst (blue) images shows the presence of live cells (A) and the capsules and absence of PI uptake (B). The white dots on the nuclei are the result of our automated counting algorithm. (C) Shows a frame of the ultra-high-speed recording during vaporization, in which the white arrows indicate internal bubbles that were formed. (D). Poration curve showing the increase in PI fluorescence intensity over time after irradiation by the pulsed laser. The black-filled red circles are the time points at which PI images (E) and (F) were recorded. The contours of the vapor bubbles are superimposed on the PI images. (G and H) Set of images showing the situation after activation. The overlay of Hoechst before and PI after laser activation shows the poration of four cells (G). The PI fluorescence signal is shown in (H). The white arrows in (G) and (H) point to the porated cells. To see this figure in color, go online.
Figure 5.
Cell viability after PMMA-hexadecane capsule activation. (A) Bright field image of the cell monolayer with the capsules floating below them. (B) Fluorescence image of calcein used to stain live cells before laser irradiation. (C) Bright field image where the bubbles are visible; internal bubbles are indicated by the white arrows. (D) Image resulting from the subtraction of the fluorescence images of calcein recorded just after and the one recorded just before laser-activation. Yellow indicates the regions of calcein signal decrease. The contours of the bubbles, extracted from the Brandaris recording (Movie S3), are superimposed on the fluorescence image. The white dashed lines, identical to those in (B), delineate the areas with >35% signal reduction. (E) Radius-time curves correspond to the vapor bubbles in (D), and (F) is the acoustic signal of the vapor bubbles. (G) Fluorescence image showing nuclei locations stained by Hoechst and cells that had taken up PI. The white dots on the nuclei are the result of our automated counting algorithm. The image was recorded at the same location as (A)–(D) after reincubation of the cells for 3 h. The reincubation allowed live cells to move slightly on the membrane, resulting in the imperfect collocation of (B) and (D) on the one hand and (G) and (H) on the other. (H) Corresponding fluorescence image of PI uptake shows the dead cells. To see this figure in color, go online.
It is interesting to note here that two different types of activation responses were observed for the capsules upon laser irradiation: a vapor bubble termed an “external” bubble and an “internal” bubble that can also be observed in Fig. 2 C. In the case of internal bubble formation, the PMMA coating does not break open and the gas probably forms by depolymerization (49) of the shell on the inner side, owing to the high temperatures that are reached in the polymer upon laser irradiation (38). Fig. 2 C shows several external bubbles, and three capsules formed internal bubbles (white arrows). We also observed that single floating capsules had a higher probability of producing internal bubbles than capsules that were close to each other.
Time-intensity curves as shown in Fig. 2 B were obtained for a collection of 21 experiments, as displayed in Fig. 3 A. The color of the curve relates to the size of the vapor bubble: the smallest bubbles (5 μm radius) are represented by light-yellow curves, whereas the largest bubbles (40 μm radius) are represented by dark-red curves. The initial poration speeds were extracted from the poration curves by means of an exponential fit and the result is displayed in Fig. 3 A, which shows a clear relationship: the larger bubbles induced faster intracellular uptake of PI. The resulting PI fluorescence rate is shown in Fig. 3 B for each bubble radius.
Figure 3.
Dynamic PI uptake. (A) Time-intensity curves of the PI signal (n = 21). The color of the curve corresponds to the size of the vapor bubble (color bar). The bubble size was measured from the Brandaris 128 recordings. (B) Initial slope extracted from the curves in (A) versus bubble size. To see this figure in color, go online.
Endothelial cell membrane poration by resomer-PFP capsules
The same poration experiments were performed on the Resomer-PFP microcapsules and a typical example is depicted in Fig. 4. The overlay of calcein and Hoechst fluorescence images is shown in Fig. 4 A and the capsules and absence of PI uptake are shown in Fig. 4 B. The vapor bubbles generated by the capsules upon laser irradiation are shown in Fig. 4 C (see also Movie S2), and the corresponding radius-time curves are shown in Fig. 4 D. Fig. 4, E and F, shows the evolution of the PI fluorescence intensity as a consequence of PI uptake by the cells that were porated, but now with background subtraction to remove the capsules and focus on the PI signal only. The contours during oscillation of the vapor bubbles are superimposed on Figs. 4, E and F; the colors correspond to the colors of the curves in Fig. 4 D. The affected cells were always in direct contact with a vapor bubble. Those bubbles created by Resomer-PFP capsules did not collapse, but remained on top of the cells. The bubbles then grew slowly over the few seconds during which the PI uptake was recorded. In Fig. 4 G, the small white arrows indicate bubble contours that partly obstructed the PI signal, and the difference between the green contour and the actual vapor bubble size in Fig. 4 H shows the bubble growth over 1 min. This growth, leading to a false-positive PI signal in the bubble core and to the partial obstruction of the PI signal from the porated cells, prevented extraction of reliable poration curves (Fig. 4 H). As said, the bubbles did not float up, which suggests that they adhered to the underlying cell layer.
Figure 4.
Cell membrane poration Resomer-PFP capsules. (A and B) Set of images showing the situation before laser irradiation. The overlay of the calcein (green) and Hoechst (blue) images shows the presence of live cells (A). The white dots on the nuclei are the result of our automated counting algorithm. The PI fluorescence signal (B) shows that no dead cells were present in the FOV. (C) Corresponding frame of the ultra-high-speed recording showing the vapor bubbles created upon irradiation by the pulsed laser. (D) Radius-time curves of the bubbles in (C). (E and F) PI fluorescence pictures at two time points showing the uptake of PI by two cells, with the contours of the vapor bubbles superimposed on them. (G and H) Set of images showing the situation after activation. The overlay of Hoechst before and PI after laser activation shows the poration of two cells (G), with the white arrows indicating partial obstruction of PI signal. The PI fluorescence signal is shown in (H). To see this figure in color, go online.
Cell viability after PMMA-hexadecane capsule activation
Control experiments (n = 10, on average 16 cells in FOV) showed cell viability over 96% in the presence of PMMA-hexadecane capsules, but without laser irradiation. When the capsules were irradiated by the laser, both in the poration and viability experiments, some capsules did not vaporize presumably because insufficient dye was encapsulated in their shell. This offered a useful control experiment (n = 3, on average 18 cells in FOV) to assess how the combination of capsules and laser irradiation affected the cells; no PI staining was observed. As a consequence, cell death by means of PI staining in the viability experiments could be directly attributed to the vapor bubbles that were formed.
The cell viability experiments after activation of PMMA-hexadecane capsules (n = 29) were performed to assess whether the produced bubbles induced cell death. Fig. 5 A shows a bright field image with the capsules floating below the cells before laser irradiation. Fig. 5 B shows a calcein image before laser irradiation, while Fig. 5 D shows the difference between the calcein image taken immediately after laser irradiation and that taken before. Comparison of the calcein signal in the white dashed circles in Fig. 5, B and D reveals an intensity decrease after laser irradiation. This decrease is indicative of poration because calcein can only leave the cell when the membrane has lost its integrity (44). The bubble contours extracted from the ultra-high-speed Brandaris recording are superimposed on Fig. 5 D. The sizes of the vapor bubbles in the viability experiments ranged from 6.5 μm in radius for the smallest, up to 40 μm for the largest bubble. In the example shown in Fig. 5, the radii were between 7.5 and 12.5 μm, and the corresponding radius-time curves (Fig. 5 E) show that the vapor bubble expanded, compressed, and stopped oscillating (see Movie S3). The lifetime of PMMA-hexadecane bubbles was found to be ∼2 μs. Fig. 5 F shows the acoustic signal emitted from the PMMA-hexadecane capsules. Fig. 5 G shows the PI fluorescence image overlayed on the Hoechst-stained cell nuclei. The cells that were stained by PI in this experiment (Fig. 5, G and H) died within 3 h after PMMA-hexadecane capsule activation. The Hoechst- and PI-stained images were both recorded after a 3-h incubation period, so the cell nuclei exactly overlapped. However, the intensity of the Hoechst signal of cells that were stained by PI was considerably lower than for the other nuclei, which were therefore not detected using our tracking algorithm. This phenomenon has recently been described in van Rooij et al. (20) and was attributed to Förster resonance energy transfer (50). The PI-stained nuclei in Fig. 5 G appear smaller than the Hoechst-stained nuclei; this shrinkage of cells and their nuclei after sonoporation has been described by others (51).
When comparing the cell nuclei locations in Fig. 5 G to those depicted in Fig. 5 D (brightest green), they do not exactly match. Our grid approach ensured that the exact same spot within the OptiCell was located, but cells could have displaced due to cell division, cellular processes involving disruption of tight junctions, or simply by sample handling, because the OptiCell had to be moved from the setup to the incubator and back.
Cell viability after resomer-PFP capsule activation
Control experiments of Resomer-PFP capsules without laser irradiation showed cell viability over 87% (n = 8, on average 20 cells in FOV). The control experiments with nonabsorbing Resomer-PFP capsules (n = 10, on average 18 cells in FOV) showed only one single cell that had taken up PI. Because no bubbles were formed, this uptake of PI was not related to an activation event.
In the cell viability experiments with Resomer-PFP capsules (n = 37), similarly sized bubbles were formed as for the PMMA-hexadecane capsules (compare Figs. 5, C and E and 6, C and E). Before and after laser irradiation, calcein fluorescence images were recorded and the white dashed circles again indicate regions of interest where calcein signal had decreased (Fig. 6, B and D), suggesting poration. In contrast to the PMMA-hexadecane vapor bubbles, the Resomer-PFP bubbles were capable of not only decreasing calcein signal, but completely depleting the signal (Fig. 6 D, bottom circle). Interestingly, Fig. 6 D shows that the PFP vapor bubble exactly colocates with the center of the ring shape of the calcein fluorescent signal. The corresponding radius-time curve (Fig. 6 E) and acoustic signal (Fig. 6 F) of this PFP vapor bubble showed sustained oscillations after the laser was turned off (see also Movie S4). Fluorescence imaging after the 3-h incubation period showed Hoechst-stained nuclei and PI staining of one cell. Due to the violent impact of the PFP vapor bubbles on the cells, parts of the cell monolayer were disrupted. It was therefore not always possible to correlate the cell nuclei (Hoechst) after laser activation (Fig. 6 G), to the location of the cells before activation. This hindered a quantitative assessment of cell viability.
Figure 6.
Cell viability after Resomer-PFP capsule activation. (A) Bright field image of the cell monolayer with the capsules on top. (B) Fluorescence image of calcein used to stain live cells before laser irradiation. (C) Bright field image showing the vapor bubbles. (D) Fluorescence image of calcein just after laser activation. The bubble contours, extracted from the Brandaris recording (Movie S4), are superimposed on the fluorescence image. The white dashed circles, identical to those in (B), show the locations where the calcein had depleted. (E) Radius-time curves correspond to the bubble contours in (B), and (F) is the acoustic signal of the vapor bubbles. (G) Fluorescence image showing nuclei locations stained by Hoechst and the white dots on the nuclei are the result of our automated counting algorithm. The image was recorded at the same location as (A)–(D) after reincubation of the cells for 3 h. (H) Corresponding fluorescence image showing PI uptake, and thus a dead cell. To see this figure in color, go online.
The stable vapor bubbles (n = 44) that were observed in the calcein fluorescence recordings (example in Fig. 6 D) ranged from 7.5 to 27 μm in radius, measured within 1 min after laser irradiation. Fig. 7, B and D, shows that the green fluorescence rings that we observed exactly colocate with the microbubbles as observed in bright field (Fig. 7, A and C). The obtained size distribution of these 44 stable bubbles is shown in Fig. 7 E.
Figure 7.
Remaining PFP bubbles. (A and C) Bright field images of laser-induced microbubbles from the Resomer-PFP capsules are shown. (B and D) Shown here are the corresponding green fluorescence images of calcein-coated microbubbles, recorded a few seconds after vaporization. (E) Bar diagram shows the size distribution of stabilized microbubbles that remained on top of the cells after laser activation of the Resomer-PFP capsules. To see this figure in color, go online.
Comparison of formulations: PMMA-hexadecane versus resomer-PFP
A summary of the poration and cell death probability is shown in Fig. 8, A and B for the PMMA-hexadecane capsules and Fig. 8, C and D for the Resomer-PFP capsules. For the PMMA-hexadecane capsules, only those that formed external bubbles were considered, because the internal bubbles never induced any cell poration and could not be sized. The bars in the figures are centered around the values on the x axis, with a bin width of 5 μm. The bar centered at 5 μm thus includes bubbles between 2.5 and 7.5 μm. The numbers above the bars represent the amount of bubbles within each bar and the height of the bar indicates the average percentage of porated or dead cells due to a single bubble. The vapor bubbles larger than 45 μm could not be accurately sized, because they often expanded beyond the FOV of the Brandaris 128 camera (175.8 × 125.9 μm).
Figure 8.
Poration and cell death probability of PMMA-hexadecane and Resomer-PFP capsules. Bar diagrams show the probability of poration and cell death per bubble as a function of its maximum radius. The numbers above the bars represent the amount of bubbles within each bar; the bin width is 5 μm centered around the value on the x axis. (A) Poration probability and (B) cell death for the PMMA-hexadecane capsules is shown. (C) Poration probability and (D) cell death for the Resomer-PFP capsules is shown. To see this figure in color, go online.
For both capsule types, larger vapor bubbles had a higher probability of porating a cell. A PMMA-hexadecane external bubble <7.5 μm had a ∼40–45% chance of cell poration and of killing a cell in its vicinity. PMMA-hexadecane bubbles >7.5 μm that were in contact with a cell resulted in 100% cell death (Fig. 8, A and B). Because the poration experiments were performed separately from the cell viability experiments, the probability of killing a cell could be higher than the poration probability. This could explain the small difference (∼10%) between the bar at 10 μm in the PMMA-hexadecane poration (Fig. 8 A) and the cell viability experiments (Fig. 8 B). At equal bubble size, Resomer-PFP bubbles had a lower probability of porating a cell than external PMMA-hexadecane bubbles. The poration probability increased for increasing bubble size, only reaching a near 100% probability for bubbles >32.5 μm radius. Cell death, however, already reached this ∼100% probability for bubbles of the same size as the PMMA external bubbles, i.e., >7.5 μm.
Acoustic characteristics
Fig. 9 A and B display the recorded acoustic traces for PMMA-hexadecane capsules and Resomer-PFP capsules, respectively, for both the poration and viability experiments. The colormap encodes the size of the vapor bubbles. The transient existence of the bubbles generated by the PMMA-hexadecane capsules induced the generation of two large positive pressure peaks, corresponding to the fast bubble expansion and its violent collapse. The time delay between these peaks thus coincides with the lifetime of the vapor bubble (<7.5 μs). The Resomer-PFP capsules, on the other hand, only generated one large positive pressure peak, owing to the higher stability of the vapor PFP bubble that prevented the violent collapse. For these capsules, prolonged low-frequency oscillations were detected (>25 μs), corresponding to the free oscillations of the created bubbles.
Figure 9.
Acoustic emissions from the microcapsules. Shown here are acoustic waveforms generated by (A) PMMA-hexadecane capsules and (B) Resomer-PFP capsules for diverse bubble sizes. (C) Initial rise of the PI intensity signal is given here, as a function of the generated peak acoustic pressure by vaporization of PMMA-hexadecane capsules, showing a clear relation. (D) Shown here are the peak pressures generated by PMMA-hexadecane (blue circles) and Resomer-PFP capsules (black circles) as functions of the bubble size. To see this figure in color, go online.
Fig. 9 C shows the initial PI influx speed into the cell as a function of the bubble radius of PMMA-hexadecane external vapor bubbles, showing faster PI influx with increasing pressure. As a consequence, therapeutic effects may be monitored remotely by a measure of the acoustic emissions, using both the frequency content and the emitted peak pressure.
Fig. 9 D displays the peak pressure that was generated as a function of the bubble size. It appears that vapor bubbles formed by both capsule types generated very similar pressure amplitudes (up to Ppeak × r = 10 Pa⋅m, i.e., ∼250 Pa for our transducer with 1.63-inch focal distance), also with a clear dependency on the bubble size: small bubbles (<30 μm) generated up to 50–100 Pa, whereas large bubbles (>30 μm) generated up to 250 Pa. One of our experiments resulted in only internal PMMA-hexadecane bubbles. The acoustic pressure emitted by these bubbles was ∼15 Pa, which would be detectable by an ultrasound scanner (52).
Discussion
Despite the capsules’ size monodispersity, their responses were found to vary from capsule to capsule. Therefore, not all capsules could be activated upon irradiation at the same laser intensity. We therefore focused on single bubble events related to cell outcome, i.e., reporting bubble sizes rather than laser intensities. Less clustering of Resomer-PFP capsules was observed than of PMMA-hexadecane capsules. More-reactive capsule clusters creating multiple bubbles that merged were treated as a single bubble.
Because the boiling point Tb of hexadecane (Tb = 286.5°C; PubChem CID:11006) is higher than that of water, the PMMA-hexadecane capsules vaporize the surrounding water (38), resulting in a water vapor bubble. In contrast, the Resomer-PFP capsules vaporize preferentially the lower boiling point PFP core (Tb = 29°C; PubChem CID 12675), which requires substantially less energy. We therefore applied higher laser intensities in our experiments on PMMA-hexadecane capsules. We verified that the highest laser intensity in the presence of nonabsorbing capsules was not sufficient to directly kill cells. However, the effect of heat deposition on the cell monolayer also needs to be addressed. From our previous theoretical work on the vaporization process of a similar type of capsule (38), we know that the maximum temperature rise in the system corresponds to the melting temperature of the polymer in the shell, which is 50°C for the Resomer-PFP capsules and 102°C for the PMMA-hexadecane capsules. After initiation of the vaporization and the resulting drop in pressure (at roughly 250 ns posttrigger), the temperature in the system is dominated by the vaporized liquid, which corresponds to 30–40°C for the Resomer-PFP capsules (PFP vapor) and 100–120°C for the PMMA-hexadecane capsules (water vapor). Therefore, the heat deposition on the cells is roughly 2.5 times higher for the PMMA-hexadecane capsules than for the Resomer-PFP capsules. These temperatures are, in principle, high enough to damage cells. However, the temperature rise is confined in time (typically a microsecond) and in space (a few micrometers, which is less than the cell size). The work of Wagner et al. (53) showed that HUVECs can withstand a heat shock of 42.5°C for 3 h. Thus, for Resomer-PFP capsules, heat deposition alone will not lead to cell death. As shown in Fig. 8, Resomer-PFP capsules and PMMA-hexadecane capsules gave similar numbers of cell death, especially for the smallest vapor bubbles that were formed, where only 40–50% of the cells in contact with either of the capsule types died. This shows a threshold with regard to the vapor bubble size that is very similar for both capsule formulations. Thus, we can only conclude that the bubble size is the determining factor, not the difference in heat deposition. It should be noted that because of the higher activation threshold, the laser intensities required to vaporize the PMMA-hexadecane capsules exceed the clinically allowed FDA limit (40).
Differences between poration and cell death by Resomer-PFP activated capsules were larger than expected. This apparent discrepancy can partly be attributed to the optical obstruction by the growing PFP bubbles, and partly by adsorption of the cell material onto the bubble (as further discussed later on). Such violent cell destruction may induce the stained genetic material to flow away in the medium, which then cannot be recorded. In addition, cell death at the start of the cell viability experiments with the Resomer-PFP capsules was found to be already slightly higher than for the poration experiments, which could have resulted in overestimation of cell death for these capsules as well.
The probability that a cell in contact with a PMMA-hexadecane or Resomer-PFP bubble died, was as high as or higher than the probability that a cell was porated. This implies that either a PMMA-hexadecane or a Resomer-PFP vapor bubble in direct contact with a cell is lethal to this cell and viable poration was not achieved. In contrast, most studies on ultrasound-mediated sonoporation report that cells can be either viably porated or irreversibly damaged (20, 21, 54). In terms of vapor bubble formation, phase-change agents that rely on acoustic vaporization more closely resemble the polymeric laser-activated microcapsules we used. These phase-change agents were smaller, and either showed mainly cell death and cell detachment (25) or both viable sonoporation and cell death (55). The smaller vapor bubbles in our experiments resulted in less and slower PI uptake (see Fig. 5). This implies that, here as well, smaller capsules may be more suitable for inducing viable poration and may open up more possibilities for drug delivery. For the formation of bubbles having diameters of ∼3 μm, the capsules should be downsized to ∼800 nm. Making them even smaller (<200 nm) would allow for extravasation and increased penetration of the capsules into the tumor tissue of interest (39). However, producing stable nanosized particles is challenging and requires optimization of the manufacturing process. Consequently, here we first focused on micrometer-sized capsules. Secondly, the resolution of our ultra-high-speed imaging system is not sufficiently high for visualization of nanocapsules. Downsizing to nanocapsules and their impact on endothelial cells and extravasation capabilities should therefore be further investigated.
For our PI uptake curves, we used a monoexponential fit, in line with Fan et al. (54) and van Rooij et al. (20). In the poration curves we extracted after activation of PMMA-hexadecane, some variation can be observed (Fig. 3 B) at the same bubble size, probably owing to differences in the location of the capsules with respect to the surrounding cells. Next to that, our method of averaging the bubble size could have influenced the relation we observed. The x axis in Fig. 3 B represents the average bubble size in the FOV. Consequently, what appears as a 10-μm bubble could either be a single bubble of 10 μm or an average of, for example, a bubble of 8 μm and one of 12 μm. Because Fig. 8 showed that larger vapor bubbles induced more damage, the larger bubble of 12 μm would result in more damage. Nonetheless, the plot clearly shows a faster PI intensity increase for larger vapor bubbles.
After laser activation of the Resomer-PFP capsules, the calcein fluorescence recordings showed a ring of bright fluorescence signal surrounding a nondissolving vapor bubble (Fig. 6 D). In previous in vitro experiments without cells, the stability of similar bubbles was investigated (56). Although these bubbles were generated acoustically, based on their results the lifetime of the Resomer-PFP bubbles was expected to be less than a few milliseconds, while the size was expected to be only several micrometers. The interesting observation that Resomer-PFP bubbles were stable for several seconds and adhered to the underlying cells could possibly be exploited for additional contrast-enhanced ultrasound imaging. Further in vitro or in vivo studies should assess whether Resomer-PFP bubbles are also stabilized in the presence of flow. This observation aids further understanding of the biophysical response of the cells to these types of vapor bubbles. Because calcein-AM only becomes fluorescent when the AM part is enzymatically cleaved off by a living cell, the ring of fluorescence has to originate from the inside of a live cell of which the membrane was disrupted. Although only calcein was visible, it is likely that other cell constituents were also incorporated within the coating of these bubbles, because the cell had completely disappeared. Human cell membranes consist mainly of lipids: phosphatidylethanolamine, phosphatidylserine, phosphatidylcholine, cholesterol, and sphingomyelin, of which phosphatidylcholines and cholesterol are the main building blocks (57, 58). Most of these phospholipids contain lipid tails with 14–24 C atoms (58). In addition, Cansell et al. (59) have shown that the main cell membrane constituents of confluent cultured human endothelial cells (EA.hy 926 line) are 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC, C16) and 1,2-distearoyl-sn-glycero-3-phosphocholine (C18). Garg et al. (60) showed the feasibility of coating microbubbles with phospholipids consisting of C16, C18, C20, C22, and C24 lipids in a 9:1 molar ratio with a lipopolymer. We and others have also shown that the use of several of these lipids resulted in stable microbubbles (61, 62, 63). In addition, coating and stabilization of bubbles with a commercially available analog of lung surfactant (Survanta; Abbott Nutrition, Columbus, OH), has been shown by Sirsi et al. (64). Survanta is primarily composed of C16 (DPPC). Moreover, DPPC is also the main component in the commercially available ultrasound contrast agent Definity (Lantheus Medical Imaging, North Billerica, MA). Because both the molecular structure of lipids and calcein is amphiphilic (PubChem CID:65079), both calcein and phospholipids could thus adsorb on the water-gas interface. The probability of the lipids and calcein adsorbing on the water-gas interface and forming a stabilizing monolayer, or coating, is extremely likely, because energetically this is the most favorable state. Recently, it has been shown that lipids can be exchanged between the cell membrane and the microbubble coating (65). The stabilization and coating of the microbubbles would be worthwhile to investigate in future studies to aid better understanding of the interaction of the vapor bubble with the cellular membrane.
The activation energy could be even lower (up to 2×) by incorporating more Sudan Red 7B in the polymer shell. This, however, resulted in a morphology change from spherical capsules to acorn- or cup-shaped particles (66). The PFP core of these particles is not completely enclosed by the Resomer, allowing easier access to the PFP core and thus a lower activation energy. Here, we also found that the poration probability of HUVECs was higher for the Resomer-PFP cups than for the Resomer-PFP capsules, consistent with the decrease in activation energy.
The work presented here aimed at studying the effect of laser-induced microbubbles on cells, in particular poration for enhanced drug delivery and cell death. In that regard, this study positions itself in the framework of photoacoustics where the penetration depth is known to be limited to a few centimeters. Consequently, only superficial tissues could be successfully treated. This represents the main limitation with respect to acoustically driven microbubbles as an alternative for enhancing drug delivery and locally controlling cell death.
Conclusions
We showed that laser-induced vaporization of polymeric microcapsules can porate and kill human endothelial cells. We studied PMMA-hexadecane capsules and Resomer-PFP capsules, of which the first formulation contained a high-boiling point oil and resulted in a higher degree of cell poration. Poration probability was already 40% for the smallest bubbles that were formed (<7.5 μm diameter), and reached 100% for the larger bubbles. Cells that were in contact with a vapor bubble and that were porated, died. Because of the lower activation threshold of Resomer-PFP capsules, this type of capsule has the highest potential to be used in vivo to induce and monitor cell death. The internal capsules produced by laser activation of PMMA-hexadecane microcapsules did not induce poration, although they still gave a detectable acoustic signal. Finally, a clear relationship between the acoustic emissions and the cell poration efficiency indicated a potential application for acoustic monitoring of the therapeutic action of such systems.
Author Contributions
G.L. and M.V. designed research. E.B. and G.V. provided new samples/reagents. G.L. and T.v.R. performed research. G.L. and T.v.R. contributed to new analytic tools. G.L. and T.v.R. analyzed data. G.L, T.v.R., I.S., N.d.J., K.K., and M.V. discussed the experiments and/or results. G.L., T.v.R., N.d.J., K.K., and M.V. wrote the manuscript.
Acknowledgments
The authors wish to gratefully acknowledge the skillful and invaluable technical support of Gert-Wim Bruggert, Martin Bos, Bas Benschop, Frits Mastik, and Robert Beurskens.
This work is supported by NanoNextNL, a micro and nanotechnology consortium of the Government of the Netherlands and 130 partners.
Editor: David Piston.
Footnotes
Guillaume Lajoinie and Tom van Rooij contributed equally to this work.
One figure and four movies are available at http://www.biophysj.org/biophysj/supplemental/S0006-3495(17)30381-8.
Supporting Material
Ultra-high-speed recording of vaporizing PMMA-hexadecane capsules in a poration experiment.
Ultra-high-speed recording of vaporizing Resomer-PFP capsules in a poration experiment.
Ultra-high-speed recording of vaporizing PMMA-hexadecane capsules in a cell viability experiment.
Ultra-high-speed recording of vaporizing Resomer-PFP capsules in a cell viability experiment.
References
- 1.International Contrast Ultrasound Society . Technical Report. ICUS; Washington, DC: 2014. CEUS around the world. [Google Scholar]
- 2.Feinstein S.B., Coll B., Thomenius K. Contrast enhanced ultrasound imaging. J. Nucl. Cardiol. 2010;17:106–115. doi: 10.1007/s12350-009-9165-y. [DOI] [PubMed] [Google Scholar]
- 3.Unnikrishnan S., Klibanov A.L. Microbubbles as ultrasound contrast agents for molecular imaging: preparation and application. AJR Am. J. Roentgenol. 2012;199:292–299. doi: 10.2214/AJR.12.8826. [DOI] [PubMed] [Google Scholar]
- 4.van Rooij T., Daeichin V., Kooiman K. Targeted ultrasound contrast agents for ultrasound molecular imaging and therapy. Int. J. Hyperthermia. 2015;31:90–106. doi: 10.3109/02656736.2014.997809. [DOI] [PubMed] [Google Scholar]
- 5.Shih R., Bardin D., Lee A.P. Flow-focusing regimes for accelerated production of monodisperse drug-loadable microbubbles toward clinical-scale applications. Lab Chip. 2013;13:4816–4826. doi: 10.1039/c3lc51016f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kooiman K., Vos H.J., de Jong N. Acoustic behavior of microbubbles and implications for drug delivery. Adv. Drug Deliv. Rev. 2014;72:28–48. doi: 10.1016/j.addr.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 7.Rychak J.J., Klibanov A.L. Nucleic acid delivery with microbubbles and ultrasound. Adv. Drug Deliv. Rev. 2014;72:82–93. doi: 10.1016/j.addr.2014.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lentacker I., De Cock I., Moonen C.T.W. Understanding ultrasound induced sonoporation: definitions and underlying mechanisms. Adv. Drug Deliv. Rev. 2014;72:49–64. doi: 10.1016/j.addr.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 9.Hernot S., Klibanov A.L. Microbubbles in ultrasound-triggered drug and gene delivery. Adv. Drug Deliv. Rev. 2008;60:1153–1166. doi: 10.1016/j.addr.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tlaxca J.L., Rychak J.J., Lawrence M.B. Ultrasound-based molecular imaging and specific gene delivery to mesenteric vasculature by endothelial adhesion molecule targeted microbubbles in a mouse model of Crohn’s disease. J. Control. Release. 2013;165:216–225. doi: 10.1016/j.jconrel.2012.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mørch Ý., Hansen R., Stenstad P.M. Nanoparticle-stabilized microbubbles for multimodal imaging and drug delivery. Contrast Media Mol. Imaging. 2015;10:356–366. doi: 10.1002/cmmi.1639. [DOI] [PubMed] [Google Scholar]
- 12.Lammers T., Koczera P., Kiessling F. Theranostic uspio-loaded microbubbles for mediating and monitoring blood-brain barrier permeation. Adv. Funct. Mater. 2015;25:36–43. doi: 10.1002/adfm.201401199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kilroy J.P., Klibanov A.L., Hossack J.A. Localized in vivo model drug delivery with intravascular ultrasound and microbubbles. Ultrasound Med. Biol. 2014;40:2458–2467. doi: 10.1016/j.ultrasmedbio.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dewitte H., Van Lint S., Lentacker I. The potential of antigen and TriMix sonoporation using mRNA-loaded microbubbles for ultrasound-triggered cancer immunotherapy. J. Control. Release. 2014;194:28–36. doi: 10.1016/j.jconrel.2014.08.011. [DOI] [PubMed] [Google Scholar]
- 15.Laing S.T., Kim H., McPherson D.D. Ultrasound-mediated delivery of echogenic immunoliposomes to porcine vascular smooth muscle cells in vivo. J. Liposome Res. 2010;20:160–167. doi: 10.3109/08982100903218918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou Y., Yang K., Deng C.X. Controlled permeation of cell membrane by single bubble acoustic cavitation. J. Control. Release. 2012;157:103–111. doi: 10.1016/j.jconrel.2011.09.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prentice P., Cuschieri A., Campbell P. Membrane disruption by optically controlled microbubble cavitation. Nat. Phys. 2005;1:107–110. [Google Scholar]
- 18.Kudo N., Okada K., Yamamoto K. Sonoporation by single-shot pulsed ultrasound with microbubbles adjacent to cells. Biophys. J. 2009;96:4866–4876. doi: 10.1016/j.bpj.2009.02.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen X., Leow R.S., Yu A.C.H. Single-site sonoporation disrupts actin cytoskeleton organization. J. R. Soc. Interface. 2014;11:20140071. doi: 10.1098/rsif.2014.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Rooij T., Skachkov I., Kooiman K. Viability of endothelial cells after ultrasound-mediated sonoporation: Influence of targeting, oscillation, and displacement of microbubbles. J. Control. Release. 2016;238:197–211. doi: 10.1016/j.jconrel.2016.07.037. [DOI] [PubMed] [Google Scholar]
- 21.Hu Y., Wan J.M.F., Yu A.C.H. Membrane perforation and recovery dynamics in microbubble-mediated sonoporation. Ultrasound Med. Biol. 2013;39:2393–2405. doi: 10.1016/j.ultrasmedbio.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 22.De Cock I., Zagato E., Lentacker I. Ultrasound and microbubble mediated drug delivery: acoustic pressure as determinant for uptake via membrane pores or endocytosis. J. Control. Release. 2015;197:20–28. doi: 10.1016/j.jconrel.2014.10.031. [DOI] [PubMed] [Google Scholar]
- 23.Kooiman K., Foppen-Harteveld M., de Jong N. Sonoporation of endothelial cells by vibrating targeted microbubbles. J. Control. Release. 2011;154:35–41. doi: 10.1016/j.jconrel.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 24.Coussios C.C., Roy R.A. Applications of acoustics and cavitation to noninvasive therapy and drug delivery. Annu. Rev. Fluid Mech. 2008;40:395–420. [Google Scholar]
- 25.Seda R., Li D.S., Bull J.L. Characterization of bioeffects on endothelial cells under acoustic droplet vaporization. Ultrasound Med. Biol. 2015;41:3241–3252. doi: 10.1016/j.ultrasmedbio.2015.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kwan J.J., Myers R., Coussios C.C. Ultrasound-propelled nanocups for drug delivery. Small. 2015;11:5305–5314. doi: 10.1002/smll.201501322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lensen D., Gelderblom E.C., van Hest J.C.M. Biodegradable polymeric microcapsules for selective ultrasound triggered drug release. Soft Matter. 2011;7:5417–5422. [Google Scholar]
- 28.Eisenbrey J.R., Hsu J., Wheatley M.A. Plasma sterilization of poly lactic acid ultrasound contrast agents: surface modification and implications for drug delivery. Ultrasound Med. Biol. 2009;35:1854–1862. doi: 10.1016/j.ultrasmedbio.2009.06.1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blanco E., Shen H., Ferrari M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 2015;33:941–951. doi: 10.1038/nbt.3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Allen T.M., Cullis P.R. Drug delivery systems: entering the mainstream. Science. 2004;303:1818–1822. doi: 10.1126/science.1095833. [DOI] [PubMed] [Google Scholar]
- 31.Kooiman K., Böhmer M.R., van Wamel A. Oil-filled polymer microcapsules for ultrasound-mediated delivery of lipophilic drugs. J. Control. Release. 2009;133:109–118. doi: 10.1016/j.jconrel.2008.09.085. [DOI] [PubMed] [Google Scholar]
- 32.Arnal B., Perez C., O’Donnell M. Sono-photoacoustic imaging of gold nanoemulsions: part I. Exposure thresholds. Photoacoustics. 2015;3:3–10. doi: 10.1016/j.pacs.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kang E., Min H.S., Kwon I.C. Nanobubbles from gas-generating polymeric nanoparticles: ultrasound imaging of living subjects. Angew. Chem. Int. Ed. Engl. 2010;49:524–528. doi: 10.1002/anie.200903841. [DOI] [PubMed] [Google Scholar]
- 34.Straub J.A., Chickering D.E., Bernstein H. Porous PLGA microparticles: AI-700, an intravenously administered ultrasound contrast agent for use in echocardiography. J. Control. Release. 2005;108:21–32. doi: 10.1016/j.jconrel.2005.07.020. [DOI] [PubMed] [Google Scholar]
- 35.Fokong S., Theek B., Lammers T. Image-guided, targeted and triggered drug delivery to tumors using polymer-based microbubbles. J. Control. Release. 2012;163:75–81. doi: 10.1016/j.jconrel.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 36.Kothapalli S.V., Daeichin V., Grishenkov D. Unique pumping-out fracturing mechanism of a polymer-shelled contrast agent: an acoustic characterization and optical visualization. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 2015;62:451–462. doi: 10.1109/TUFFC.2014.006732. [DOI] [PubMed] [Google Scholar]
- 37.Wilson K., Homan K., Emelianov S. Biomedical photoacoustics beyond thermal expansion using triggered nanodroplet vaporization for contrast-enhanced imaging. Nat. Commun. 2012;3:618. doi: 10.1038/ncomms1627. [DOI] [PubMed] [Google Scholar]
- 38.Lajoinie G., Gelderblom E., Versluis M. Ultrafast vapourization dynamics of laser-activated polymeric microcapsules. Nat. Commun. 2014;5:3671. doi: 10.1038/ncomms4671. [DOI] [PubMed] [Google Scholar]
- 39.Hobbs S.K., Monsky W.L., Jain R.K. Regulation of transport pathways in tumor vessels: role of tumor type and microenvironment. Proc. Natl. Acad. Sci. USA. 1998;95:4607–4612. doi: 10.1073/pnas.95.8.4607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Standard IEC . International Electrotechnical Commission; Geneva, Switzerland: 2001. Safety of Laser Products—Part 1: Equipment Classification, Requirements and User’s Guide. [Google Scholar]
- 41.Loxley A., Vincent B. Preparation of Poly(methylmethacrylate) microcapsules with liquid cores. J. Colloid Interface Sci. 1998;208:49–62. doi: 10.1006/jcis.1998.5698. [DOI] [PubMed] [Google Scholar]
- 42.Chin C.T., Lancée C., Lohse D. Brandaris 128: a digital 25 million frames per second camera with 128 highly sensitive frames. Rev. Sci. Instrum. 2003;74:5026–5034. [Google Scholar]
- 43.Gelderblom, E. C., …, M. Versluis, Brandaris 128 ultra-high-speed imaging facility: 10 years of operation, updates, and enhanced features. Rev. Sci. Instr. 83:103706. [DOI] [PubMed]
- 44.Schlicher R.K., Radhakrishna H., Prausnitz M.R. Mechanism of intracellular delivery by acoustic cavitation. Ultrasound Med. Biol. 2006;32:915–924. doi: 10.1016/j.ultrasmedbio.2006.02.1416. [DOI] [PubMed] [Google Scholar]
- 45.Brana C., Benham C., Sundstrom L. A method for characterising cell death in vitro by combining propidium iodide staining with immunohistochemistry. Brain Res. Brain Res. Protoc. 2002;10:109–114. doi: 10.1016/s1385-299x(02)00201-5. [DOI] [PubMed] [Google Scholar]
- 46.Steinkamp J.A., Lehnert B.E., Lehnert N.M. Discrimination of damaged/dead cells by propidium iodide uptake in immunofluorescently labeled populations analyzed by phase-sensitive flow cytometry. J. Immunol. Methods. 1999;226:59–70. doi: 10.1016/s0022-1759(99)00053-8. [DOI] [PubMed] [Google Scholar]
- 47.Klema V.C., Laub A.J. The singular value decomposition—its computation and some applications. IEEE Trans. Automat. Contr. 1980;25:164–176. [Google Scholar]
- 48.Suzuki T., Fujikura K., Takata K. DNA staining for fluorescence and laser confocal microscopy. J. Histochem. Cytochem. 1997;45:49–53. doi: 10.1177/002215549704500107. [DOI] [PubMed] [Google Scholar]
- 49.Smolders K., Baeyens J. Thermal degradation of PMMA in fluidised beds. Waste Manag. 2004;24:849–857. doi: 10.1016/j.wasman.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 50.Wu P., Brand L. Resonance energy transfer: methods and applications. Anal. Biochem. 1994;218:1–13. doi: 10.1006/abio.1994.1134. [DOI] [PubMed] [Google Scholar]
- 51.Chen X., Wan J.M.F., Yu A.C.H. Sonoporation as a cellular stress: induction of morphological repression and developmental delays. Ultrasound Med. Biol. 2013;39:1075–1086. doi: 10.1016/j.ultrasmedbio.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 52.van Neer P.L., Matte G., de Jong N. Super-harmonic imaging: development of an interleaved phased-array transducer. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 2010;57:455–468. doi: 10.1109/TUFFC.2010.1426. [DOI] [PubMed] [Google Scholar]
- 53.Wagner M., Hermanns I., Kirkpatrick C.J. Induction of stress proteins in human endothelial cells by heavy metal ions and heat shock. Am. J. Physiol. 1999;277:L1026–L1033. doi: 10.1152/ajplung.1999.277.5.L1026. [DOI] [PubMed] [Google Scholar]
- 54.Fan Z., Liu H., Deng C.X. Spatiotemporally controlled single cell sonoporation. Proc. Natl. Acad. Sci. USA. 2012;109:16486–16491. doi: 10.1073/pnas.1208198109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Burgess M.T., Porter T.M. Acoustic cavitation-mediated delivery of small interfering ribonucleic acids with phase-shift nano-emulsions. Ultrasound Med. Biol. 2015;41:2191–2201. doi: 10.1016/j.ultrasmedbio.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Reznik N., Shpak O., Burns P.N. The efficiency and stability of bubble formation by acoustic vaporization of submicron perfluorocarbon droplets. Ultrasonics. 2013;53:1368–1376. doi: 10.1016/j.ultras.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 57.Garret R.H., Grisham C.M. Brooks/Cole/Cengage Learning; Boston, MA: 2005. Biochemistry. [Google Scholar]
- 58.Alberts B., Johnson A., Walter P. Garland Science/Taylor & Francis; New York: 2002. Molecular Biology of the Cell. [Google Scholar]
- 59.Cansell M., Gouygou J.-P., Letourneur D. Lipid composition of cultured endothelial cells in relation to their growth. Lipids. 1997;32:39–44. doi: 10.1007/s11745-997-0006-3. [DOI] [PubMed] [Google Scholar]
- 60.Garg S., Thomas A.A., Borden M.A. The effect of lipid monolayer in-plane rigidity on in vivo microbubble circulation persistence. Biomaterials. 2013;34:6862–6870. doi: 10.1016/j.biomaterials.2013.05.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Daeichin V., van Rooij T., Kooiman K. Microbubble composition and preparation for high-frequency contrast-enhanced ultrasound imaging: in vitro and in vivo evaluation. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 2017;64:555–567. doi: 10.1109/TUFFC.2016.2640342. [DOI] [PubMed] [Google Scholar]
- 62.Kim D.H., Costello M.J., Needham D. Mechanical properties and microstructure of polycrystalline phospholipid monolayer shells: novel solid microparticles. Langmuir. 2003;19:8455–8466. [Google Scholar]
- 63.Zhao S., Borden M., Dayton P.A. Radiation-force assisted targeting facilitates ultrasonic molecular imaging. Mol. Imaging. 2004;3:135–148. doi: 10.1162/1535350042380317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sirsi S.R., Fung C., Borden M.A. Lung surfactant microbubbles increase lipophilic drug payload for ultrasound-targeted delivery. Theranostics. 2013;3:409–419. doi: 10.7150/thno.5616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Carugo D., Aron M., Stride E. Modulation of the molecular arrangement in artificial and biological membranes by phospholipid-shelled microbubbles. Biomaterials. 2017;113:105–117. doi: 10.1016/j.biomaterials.2016.10.034. [DOI] [PubMed] [Google Scholar]
- 66.Pisani E., Tsapis N., Fattal E. Polymeric nano/microcapsules of liquid perfluorocarbons for ultrasonic imaging: physical characterization. Langmuir. 2006;22:4397–4402. doi: 10.1021/la0601455. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Ultra-high-speed recording of vaporizing PMMA-hexadecane capsules in a poration experiment.
Ultra-high-speed recording of vaporizing Resomer-PFP capsules in a poration experiment.
Ultra-high-speed recording of vaporizing PMMA-hexadecane capsules in a cell viability experiment.
Ultra-high-speed recording of vaporizing Resomer-PFP capsules in a cell viability experiment.