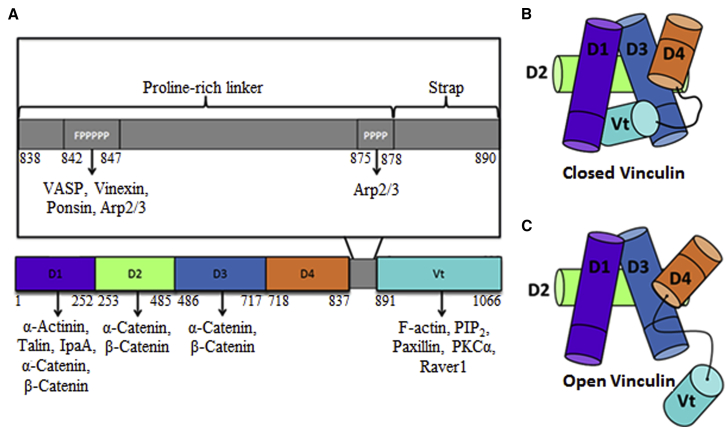
Figure 1.
Vinculin structural features, motifs, and binding partners. (A) Given here is a schematic of vinculin outlining the domains, motifs, and major binding partners of vinculin. Above the overall schematic of vinculin, a zoom-in on the proline-rich linker and strap is shown. Two motifs on the Proline-rich linker, the FPPPPP motif and the PPPP motif, are necessary for binding to various vinculin binding partners. The structures of closed vinculin (B) and open vinculin (C) are also depicted above. Interactions among domains D1, D3, and D4 with Vt block the binding sites of vinculin for many of its binding partners, rendering vinculin in an autoinhibited state. Intracellular or extracellular forces promote the release of the Vt domain from the vinculin head domain, inducing vinculin activation and binding to its binding partners. To see this figure in color, go online.