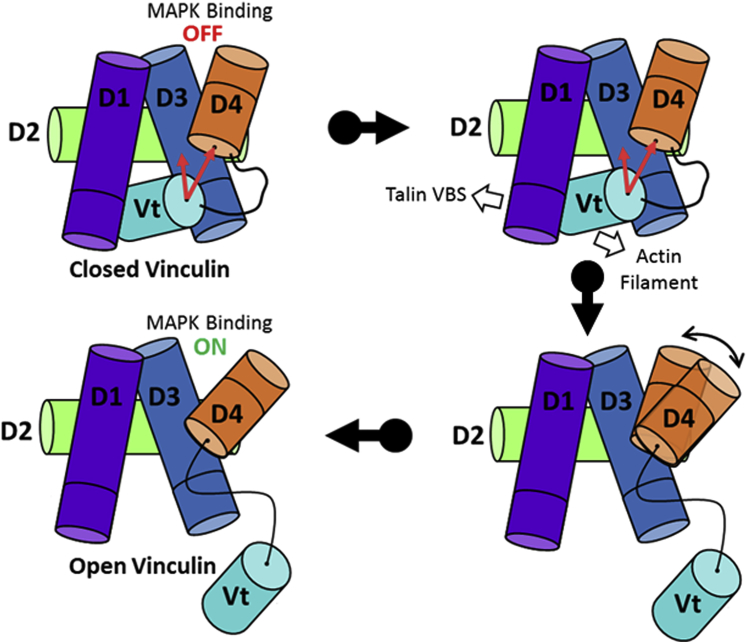
Figure 6.
A model for the mechanoselectivity of vinculin-MAPK1 binding. Vinculin domains D1, D2, D3, D4, and Vt are shown in violet, lime, blue, orange, and cyan, respectively. The D3–D4 cleft of closed vinculin is stabilized in a MAPK1-inaccessible state through simultaneous interactions between D3 and D4 with Vt. Upon force-dependent talin engagement with vinculin, the Vt domain is released from the head domain, relieving its interaction with D3 and D4. The release of Vt from the head domain is proposed to increase the flexibility of the D3–D4 interface, releasing the steric constraints and permitting MAPK1 binding. To see this figure in color, go online.