Abstract
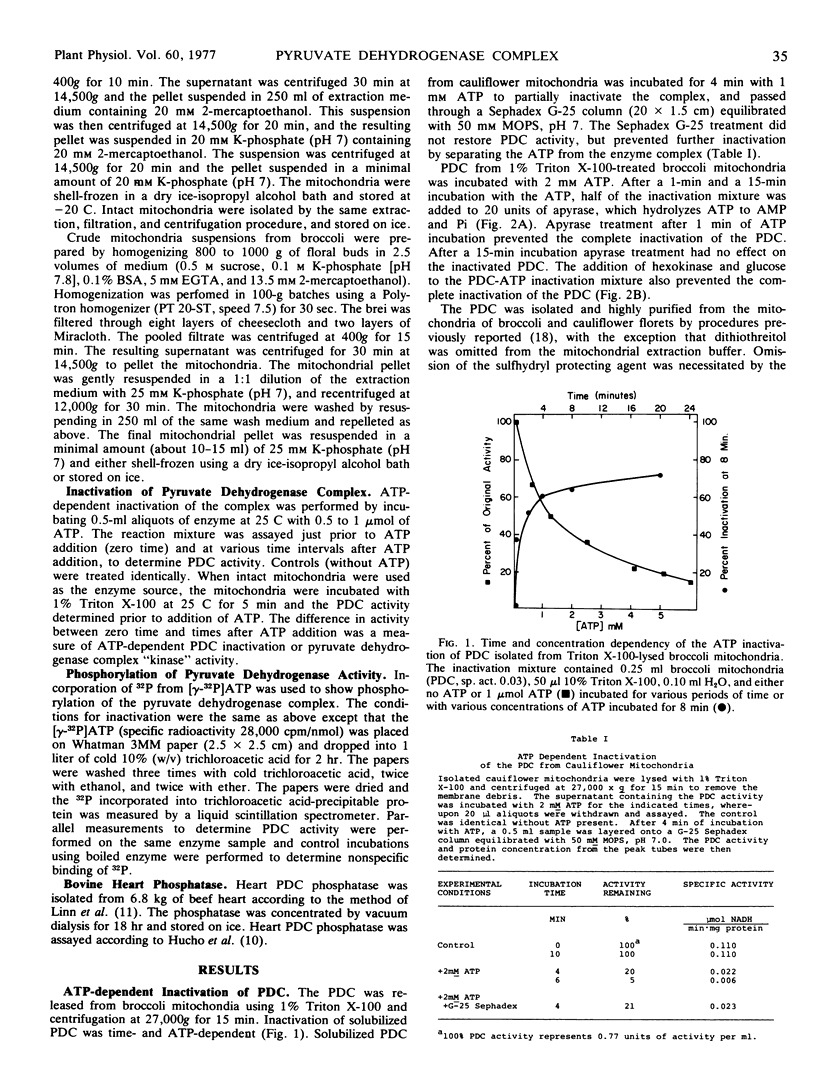
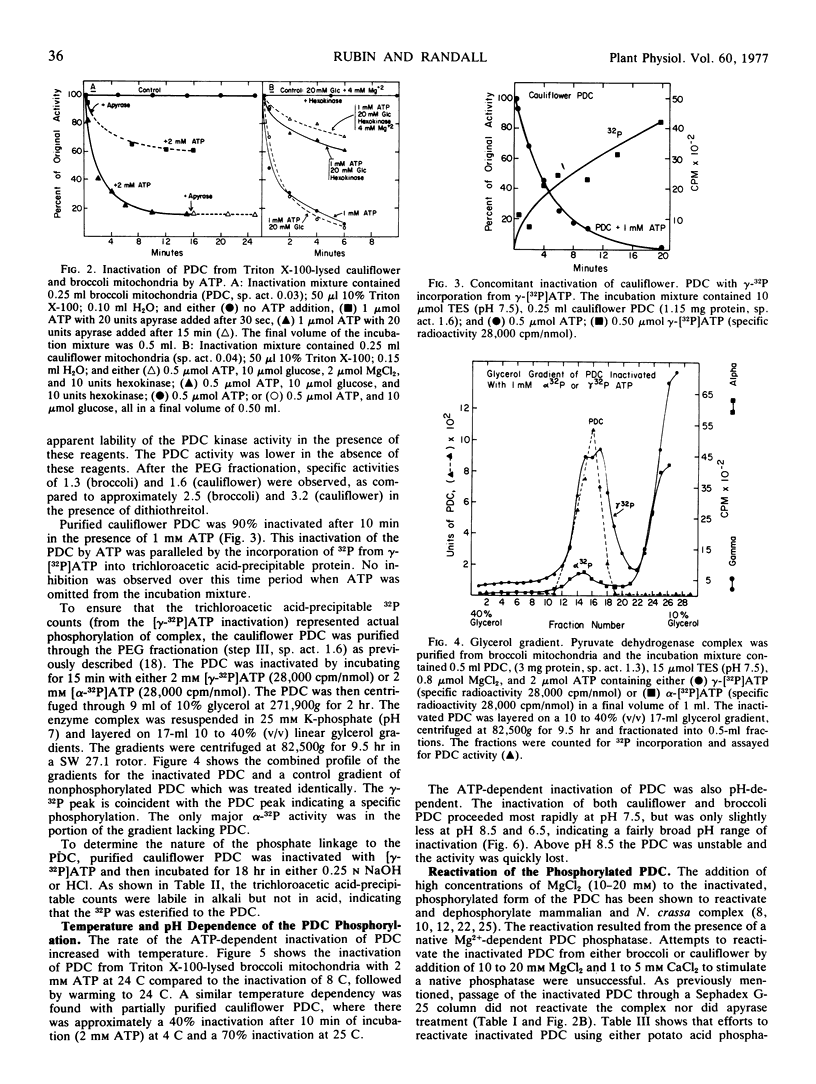
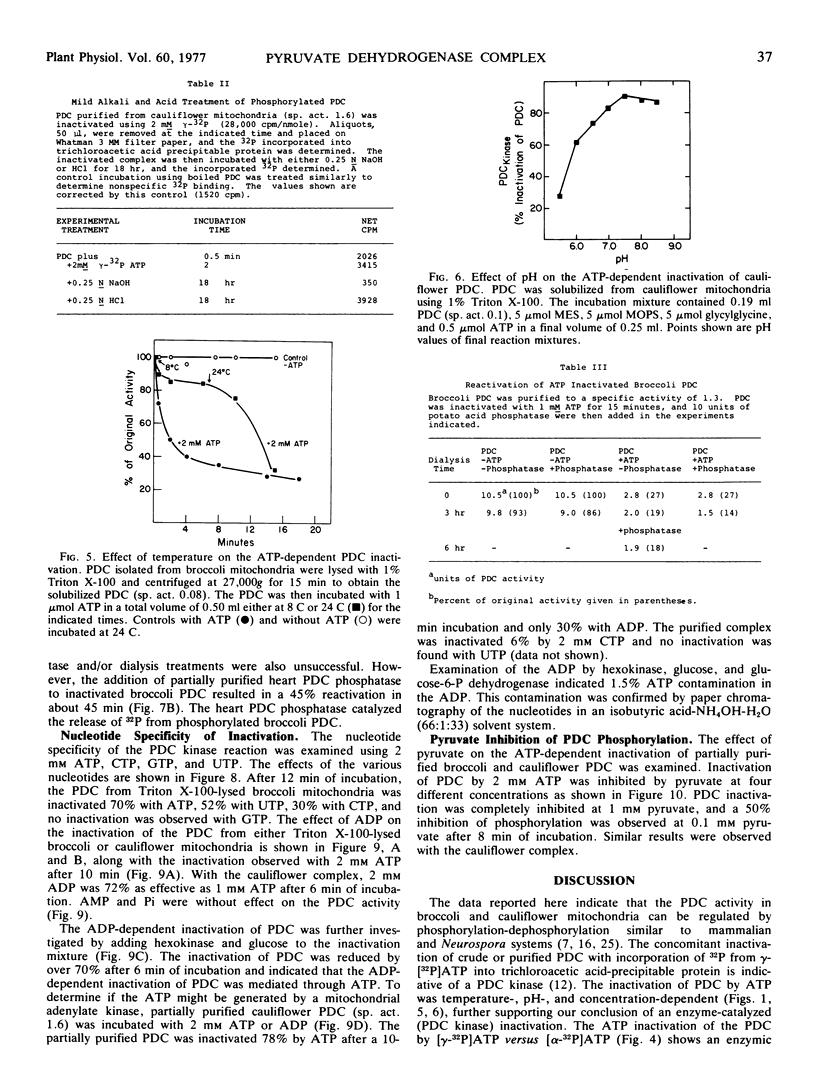
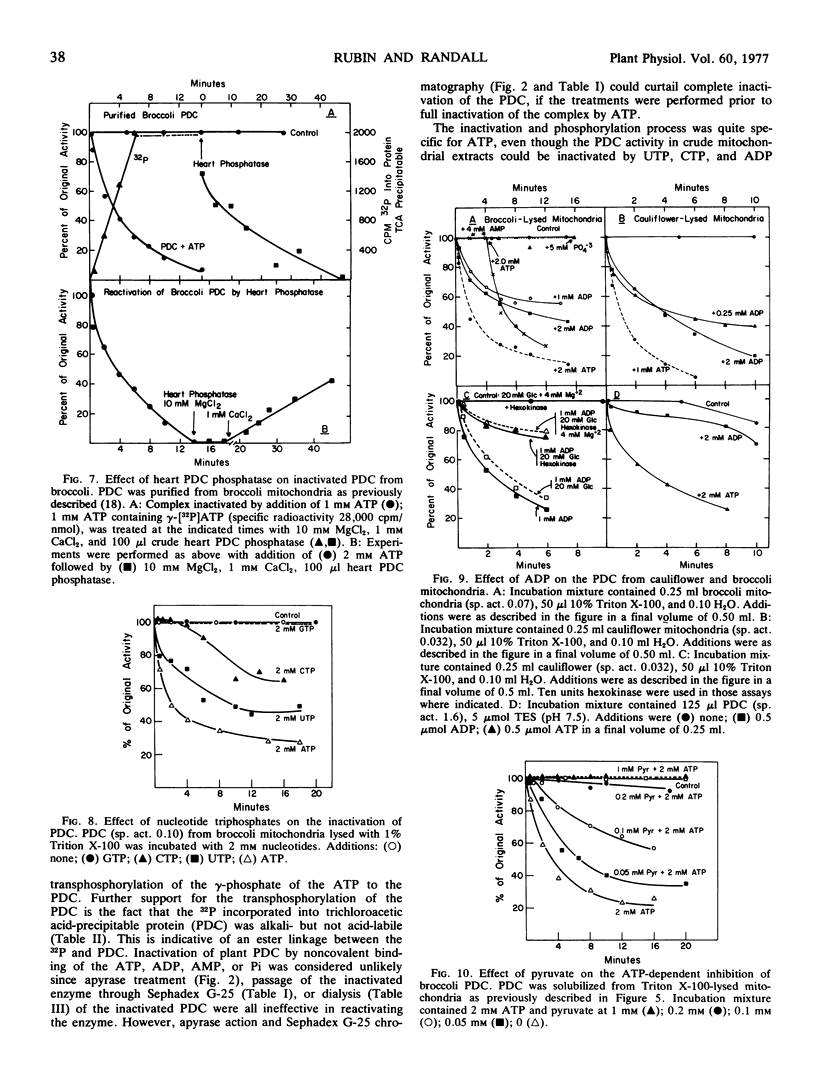
The ATP-dependent inactivation of the pyruvate dehydrogenase complex (PDC) was examined using ruptured mitochondria and partially purified pyruvate dehydrogenase complex isolated from broccoli and cauliflower (Brassica oleracea) bud mitochondria. The ATP-dependent inactivation was temperature- and pH-dependent. [32P]ATP experiments show a specific transphosphorylation of the γ-PO4 of ATP to the complex. The phosphate attached to the PDC was labile under mild alkaline but not under mild acidic conditions. The inactivated-phosphorylated PDC was not reactivated by 20 mm MgCl2, dialysis, Sephadex G-25 treatment, apyrase action, or potato acid phosphatase action. However, partially purified bovine heart PDC phosphatase catalyzed the reactivation and dephosphorylation of the isolated plant PDC. The ATP-dependent inactivation-phosphorylation of the PDC was inhibited by pyruvate. It is concluded that the ATP-dependent inactivation-phosphorylation of broccoli and cauliflower mitochondrial PDC is catalyzed by a PDC kinase. It is further concluded that the PDC from broccoli and cauliflower mitochondria is capable of interconversion between an active (dephosphorylated) and an inactive (phosphorylated) form.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bingham E. W., Farrell H. M., Jr Removal of phosphate groups from casein with potato acid phosphatase. Biochim Biophys Acta. 1976 Apr 8;429(2):448–460. doi: 10.1016/0005-2744(76)90293-x. [DOI] [PubMed] [Google Scholar]
- Bowman E. J., Ikuma H. Regulation of malate oxidation in isolated mung bean mitochondria: I. Effects of oxaloacetate, pyruvate, and thiamine pyrophosphate. Plant Physiol. 1976 Sep;58(3):433–437. doi: 10.1104/pp.58.3.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bresters T. W., de Abreu R. A., de Kok A., Visser J., Veeger C. The pyruvate-dehydrogenase complex from Azotobacter vinelandii. Eur J Biochem. 1975 Nov 15;59(2):335–345. doi: 10.1111/j.1432-1033.1975.tb02460.x. [DOI] [PubMed] [Google Scholar]
- Chiang P. K., Sacktor B. Control of pyruvate dehydrogenase activity in intact cardiac mitochondria. Regulation of the inactivation and activation of the dehydrogenase. J Biol Chem. 1975 May 10;250(9):3399–3408. [PubMed] [Google Scholar]
- Cooper R. H., Randle P. J., Denton R. M. Regulation of heart muscle pyruvate dehydrogenase kinase. Biochem J. 1974 Dec;143(3):625–641. doi: 10.1042/bj1430625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crompton M., Laties G. G. The regulatory function of potato pyruvate dehydrogenase. Arch Biochem Biophys. 1971 Mar;143(1):143–150. doi: 10.1016/0003-9861(71)90194-9. [DOI] [PubMed] [Google Scholar]
- Denton R. M., Randle P. J., Martin B. R. Stimulation by calcium ions of pyruvate dehydrogenase phosphate phosphatase. Biochem J. 1972 Jun;128(1):161–163. doi: 10.1042/bj1280161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hucho F., Randall D. D., Roche T. E., Burgett M. W., Pelley J. W., Reed L. J. -Keto acid dehydrogenase complexes. XVII. Kinetic and regulatory properties of pyruvate dehydrogenase kinase and pyruvate dehydrogenase phosphatase from bovine kidney and heart. Arch Biochem Biophys. 1972 Jul;151(1):328–340. doi: 10.1016/0003-9861(72)90504-8. [DOI] [PubMed] [Google Scholar]
- Hucho F. The pyruvate dehydrogenase multienzyme complex. Angew Chem Int Ed Engl. 1975 Sep;14(9):591–601. doi: 10.1002/anie.197505911. [DOI] [PubMed] [Google Scholar]
- Jackson A. O., Larkins B. A. Influence of Ionic Strength, pH, and Chelation of Divalent Metals on Isolation of Polyribosomes from Tobacco Leaves. Plant Physiol. 1976 Jan;57(1):5–10. doi: 10.1104/pp.57.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Linn T. C., Pelley J. W., Pettit F. H., Hucho F., Randall D. D., Reed L. J. -Keto acid dehydrogenase complexes. XV. Purification and properties of the component enzymes of the pyruvate dehydrogenase complexes from bovine kidney and heart. Arch Biochem Biophys. 1972 Feb;148(2):327–342. doi: 10.1016/0003-9861(72)90151-8. [DOI] [PubMed] [Google Scholar]
- Linn T. C., Pettit F. H., Reed L. J. Alpha-keto acid dehydrogenase complexes. X. Regulation of the activity of the pyruvate dehydrogenase complex from beef kidney mitochondria by phosphorylation and dephosphorylation. Proc Natl Acad Sci U S A. 1969 Jan;62(1):234–241. doi: 10.1073/pnas.62.1.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh H. V., Galmiche J. M., Gibbs M. Effect of Light on the Tricarboxylic Acid Cycle in Scenedesmus. Plant Physiol. 1965 Nov;40(6):1013–1022. doi: 10.1104/pp.40.6.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin C. S., Rosen O. M. Protein phosphorylation. Annu Rev Biochem. 1975;44:831–887. doi: 10.1146/annurev.bi.44.070175.004151. [DOI] [PubMed] [Google Scholar]
- Rubin P. M., Randall D. D. Purification and characterization of pyruvate dehydrogenase complex from borccoli floral buds. Arch Biochem Biophys. 1977 Jan 30;178(2):342–349. doi: 10.1016/0003-9861(77)90202-8. [DOI] [PubMed] [Google Scholar]
- Segal H. L. Enzymatic interconversion of active and inactive forms of enzymes. Science. 1973 Apr 6;180(4081):25–32. doi: 10.1126/science.180.4081.25. [DOI] [PubMed] [Google Scholar]
- Severson D. L., Denton R. M., Pask H. T., Randle P. J. Calcium and magnesium ions as effectors of adipose-tissue pyruvate dehydrogenase phosphate phosphatase. Biochem J. 1974 May;140(2):225–237. doi: 10.1042/bj1400225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieland O. H., Hartmann U., Siess E. A. Neurospora crassa pyruvate dehydrogenase: interconversion by phosphorylation and dephosphorylation. FEBS Lett. 1972 Nov 1;27(2):240–244. doi: 10.1016/0014-5793(72)80630-6. [DOI] [PubMed] [Google Scholar]


