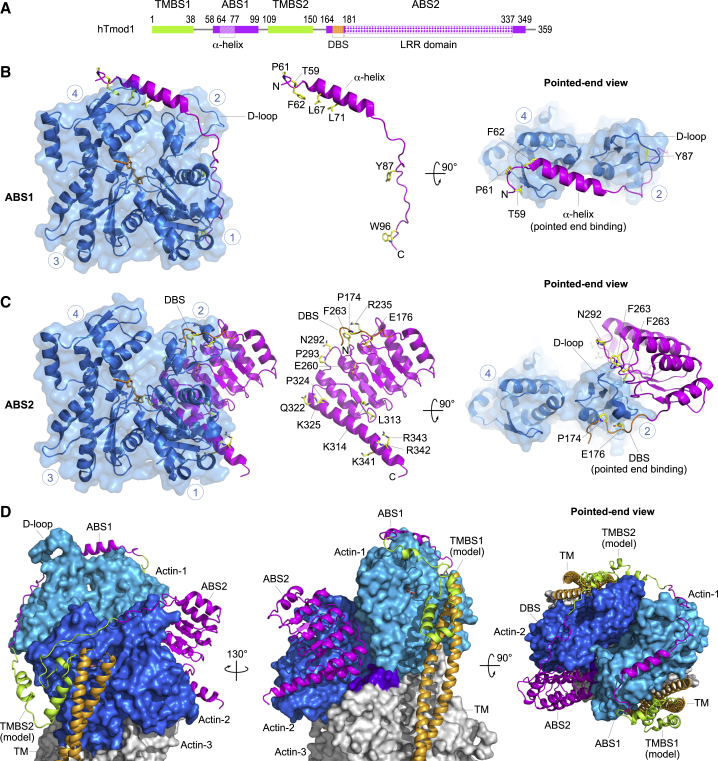
Figure 3.
Tmod structures and pointed-end capping model. (A) Domain diagram of human Tmod1 (the other three Tmod isoforms have a very similar domain architecture). Domains are drawn to scale and colored and numbered according to Fig. 1. (B and C) Structures of Tmod ABS1 and ABS2 bound to actin (5), showing frontal and pointed end views. ABS1 and ABS2 are also shown separately in the middle, highlighting amino acids that have been mutated and shown to contribute toward Tmod’s pointed end-capping activity (5,31,38,44,55) (human Tmod1 numbering). Circled blue numbers indicate the four subdomains of actin. N and C denote the N- and C-terminal ends of the bound Tmod domains. (D) Model of Tmod at the pointed end is given (5). The structures of ABS1 and ABS2 in complex with actin were superimposed, respectively, onto the first and second subunits (marine and blue) of the filament structure (52). At this location, ABS2 also contacts the first and third (purple-colored surface) actin subunits of the filament. The structure of the two TM-binding sites (green) is unknown, and a tentative model was generated based on secondary structure prediction and energy minimization (5). TM is shown in the blocked state, which it assumes when bound to the filament with Ca2+-free troponin (53, 111). Of note, this model is consistent with TM’s ability to explore all three states on the filament (blocked, closed, and open) (53,54), without generating steric clashes with Tmod at the pointed end. To see this figure in color, go online.