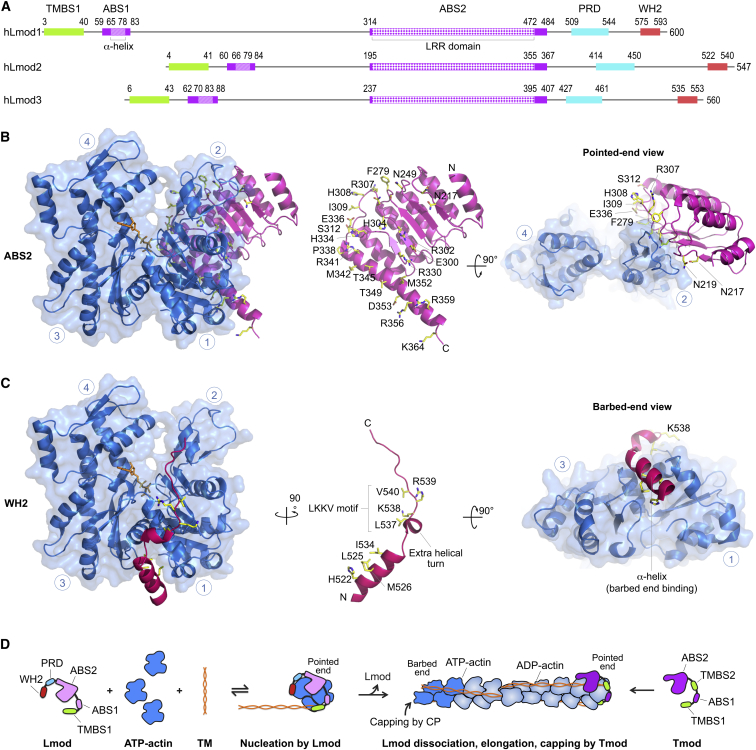
Figure 4.
Lmod structures and nucleation model. (A) Domain diagram of the three Lmod isoforms expressed in humans. The diagrams are centered on ABS2, the most highly conserved region among isoforms. Domains are drawn to scale and colored and numbered according to Fig. 1. (B–C) Shown here are actin (blue) complexes with ABS2 (magenta) and the WH2 domain (red) from the structure of Lmod2 in complex with actin (PDB: 4RWT) (59). In addition to the frontal view, pointed end and barbed end views are shown for ABD2 and the WH2 domain, respectively. The two domains are also shown separately in the middle, highlighting amino acids that participate in interactions with actin (human Lmod2 numbering). Circled blue numbers indicate the four subdomains of actin. N and C denote the N- and C-terminal ends of the Lmod domains. Note that Lmod lacks DBS, which in Tmod interacts with the D-loop of actin (compare Figs. 2B, right, and 3C, right). (D) Shown here is the proposed nucleation mechanism of Lmod. Lmod contains two major actin-binding sites (ABS2 and the WH2 domain) and one tropomyosin-binding site, TMBS1. Although ABS1, present in all Tmod isoforms, is not well conserved in Lmods, it was recently proposed that a functional ABS1 may exist in Lmod (41), albeit N-terminally shifted compared to Tmods (see Fig. 1). Through its actin- and TM-binding sites, Lmod recruits at least three actin subunits and one TM molecule to form a polymerization nucleus. Lmod likely dissociates from the nucleus when it begins to elongate, which can be triggered by several factors: steric hindrance of the WH2 domain with actin subunits adding at the barbed end of the original nucleus and the lack of pointed-end capping elements present in Tmod, including most of ABS1, TMBS2, and DBS, which together contribute to the very high affinity of Tmod for pointed ends. Lmod dissociation would free both the pointed and barbed ends of the polymerization nucleus, allowing it to elongate while also freeing the way for Tmod to bind and cap the pointed end. To see this figure in color, go online.