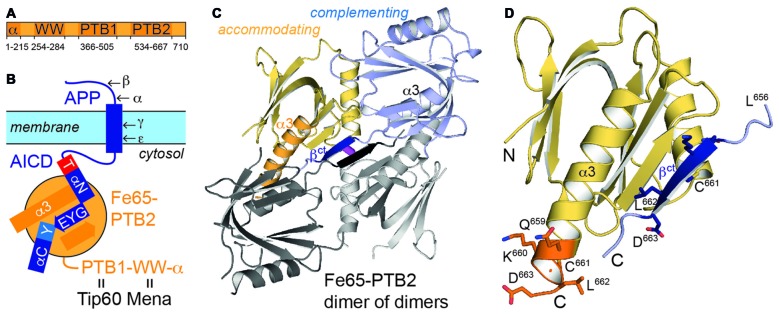
Figure 1.
Fe65 and amyloid precursor protein (APP). (A) Domain architecture of human Fe65 with numbering of domain boundaries. (B) Schematic for Fe65-mediated APP-signaling by the APP intracellular domain (AICD)/Fe65-phosphotyrosine-binding domains 2 (PTB2) complex at the cell membrane. Structural details for the interaction are depicted as follows: αN and αC: AICD helices; T and Y: AICD sequence fingerprints (T: T668PEE, Y: N684PTY) as part of AICD helices, GYE: AICD region involved in β-augmentation with Fe65-PTB2. APP-cleavage sites by secretases are indicated by Greek symbols. (C) X-ray structure of the Fe65-PTB2 dimer of dimers. The dimer is constituted by a “complementing” subunit (blue) with a transition of the C-terminus to strand βct (dark blue), while the “accommodating” subunit (yellow) contains the entire helix α3 (orange). The second dimer symmetrically attached by β-augmentation is shown with gray subunits. The central disulfide bond connecting the dimer of dimers is shown in magenta. (D) Close-up on the C-terminal Fe65-transition. According regions (L656-D663) of the complementing (dark blue, βct) and accommodating (orange, α3) subunits are given with side chains and numbering.