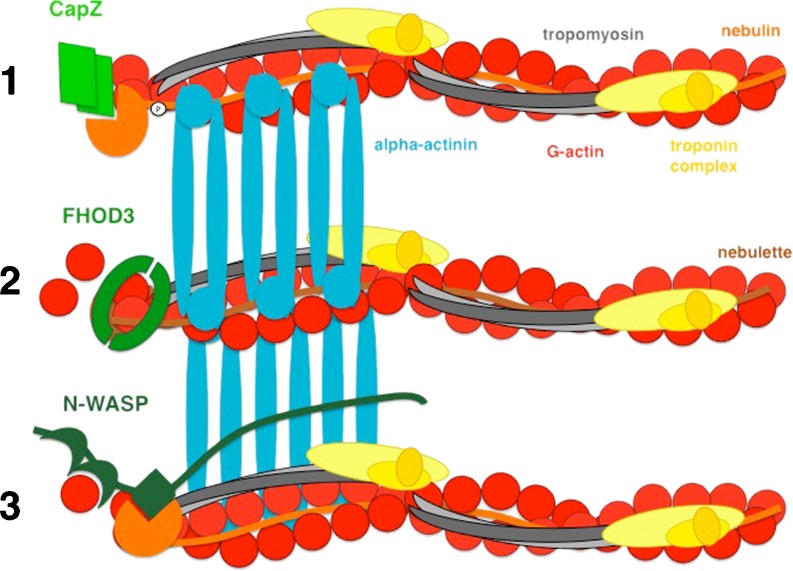
Fig. 2.
Three different models for the regulation of actin filament integrity and assembly at the barbed end at the Z-disc in myocytes. At resting state, actin filaments are capped by CapZ via its interaction with nebulin’s C-terminal SH3 domain (scenario 1) (Schafer et al. 1995; Witt et al. 2006), or potentially by FHOD3 (scenario 2) (Iskratsch and Ehler 2011). Upon activation e.g. while adapting to cell culture conditions or following actin filament depolymerisation, FHOD3 can switch to a mode that supports actin filament formation (Iskratsch et al. 2010). In skeletal muscle, nebulin’s SH3 domain can serve to activate N-WASP, which in turn stimulates actin filament formation via a hithertho not defined actin elongation factor (scenario 3) (Takano et al. 2010). Actin is represented in red, alpha-actinin in turquoise, CapZ in green, nebulin in orange, nebulette in orange, tropomyosin in grey and the troponin complex in yellow, CapZ, FHOD3 and N-WASP are represented in different shades of green. This is an extremely minimalistic view of the Z-disc, since the plethora of proteins that localises to that part of the sarcomere has been left out for reasons of simplification. For recent excellent reviews on the Z-disc, see Faulkner et al. (2001) and Frank et al. (2006); for a recent 3D model of the Z-disc, see Waardenberg et al. (2011)