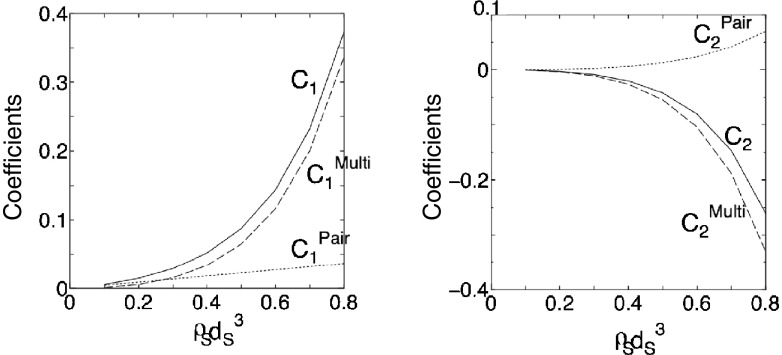
Fig. 5.
Dependence of C 1 Pair, C 1 Multi, C 2 Pair, and C 2 Multi on ρ S d S 3 for hard-sphere solvent. C 1 Pair = C 1 for the pair correlation component of −S V/k B (C 1 Pair = ρ S), C 1 Multi = C 1 for the many-body correlation component of −S V/k B, C 2 Pair = C 2 for the pair correlation component of −S V/k B, and C 2 Multi = C 2 for the many-body correlation component of −S V/k B. C 1 = C 1 Pair + C 1 Multi and C 2 = C 2 Pair + C 2 Multi. C 1 and C 2 are the first and second principal coefficients in the morphometric forms. S V, k B, ρ S, and d S are the solvation entropy, Boltzmann constant, number density of bulk solvent, and diameter of solvent molecules, respectively. If the Asakura–Oosawa theory is applied to the present system, C 1 Pair = ρ S, C 1 Multi = 0, C 2 Pair = 0, and C 2 Multi = 0