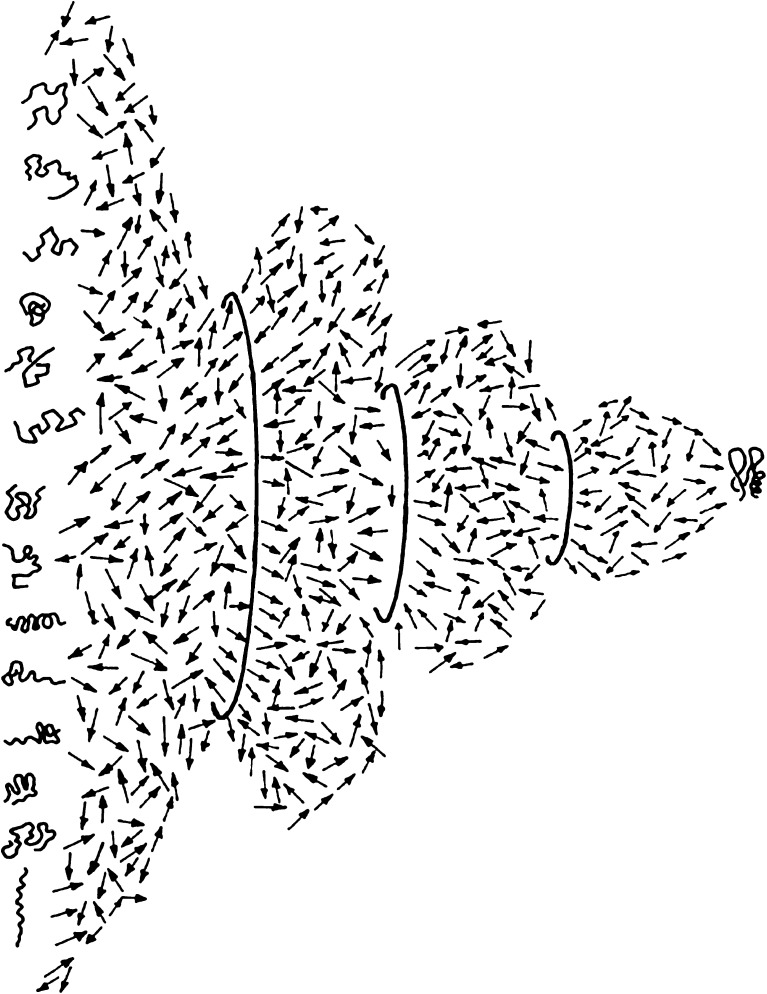
Fig. 1.
Structural view of the folding transition. A two-dimensional slice in the multidimensional conformational hyperspace of a refolding protein molecule. The transition between the large ensemble of disordered chain conformations and the small ensemble of native chain folds is based on purely stochastic thermal motions of all the chain segments driven by the thermal energy of the solvent. The directed folding transition, which is based on these elementary stochastic steps, is achieved by means of the gradual addition of conformational constraints, represented by the circles of smaller and smaller diameters, which represent the reduction of the conformational space. These constraints consist of local and non-local cross links between chain elements that reduce the degrees of freedom of the backbone and the side chains. The search for the interactions that impose these restrictions is a major goal of folding research aimed at deciphering the relationship between the genetic (sequence) information and the formation of the folded state of the protein molecules (reproduced with permission)