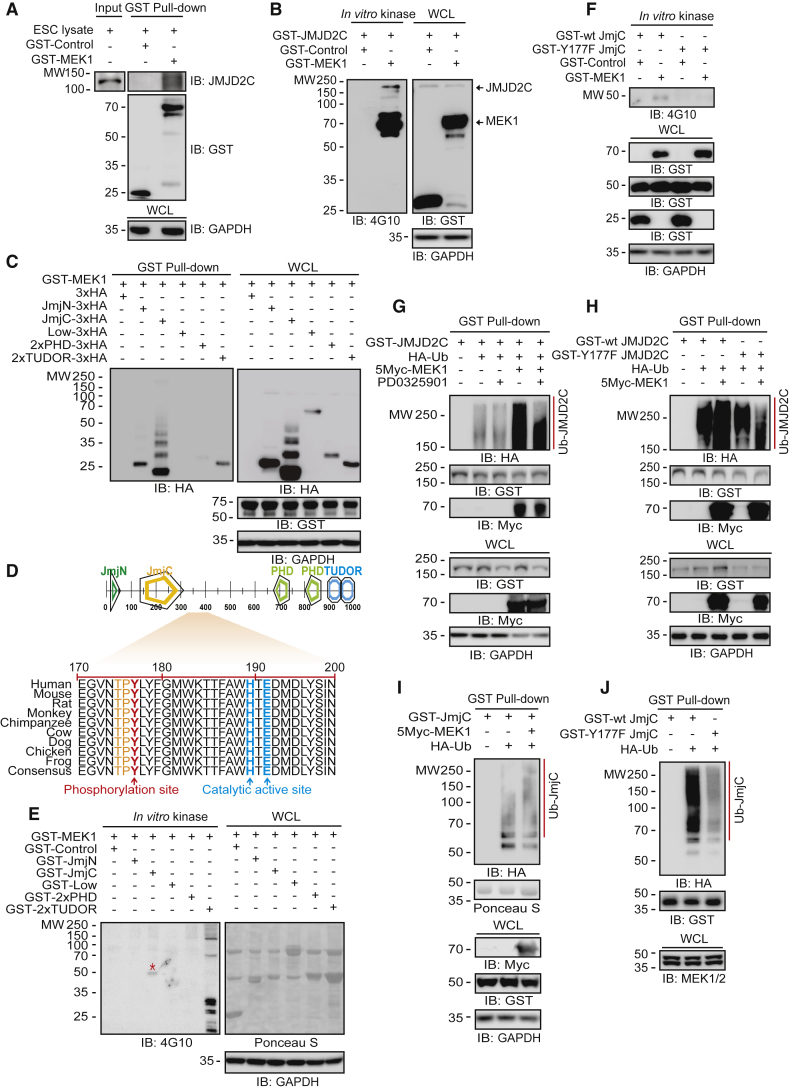
Figure 2.
MEK1 Interacts with and Phosphorylates JMJD2C, Which Undergoes Phosphorylation-Dependent Degradation through the Ubiquitin-Pproteasome Pathway
(A) GST pull-down assay shows that MEK1 associates with endogenous JMJD2C.
(B) An in vitro MEK1 kinase assay performed with mouse anti-phosphotyrosine-specific 4G10 antibody shows that MEK1 phosphorylates tyrosine residues of JMJD2C.
(C) MEK1 binds to the JmjN, JmjC, 2×PHD, and 2×TUDOR domains in JMJD2C.
(D) The Y177 residue in JMJD2C is a putative site for phosphorylation by MEK1.
(E and F) In vitro MEK1 kinase assays show that (E) MEK1 phosphorylates the JmjC domain in JMJD2C, and (F) MEK1 phosphorylates wild-type JmjC but not Y177F mutant JmjC. The red asterisk indicates a phosphorylated domain by MEK1.
(G) MEK1 expression increases JMJD2C ubiquitination, but MEKi by PD0325901 suppresses JMJD2C ubiquitination.
(H) MEK1 increases the ubiquitination of wild-type JMJD2C but not Y177F mutant JMJD2C.
(I) MEK1 increases wild-type JmjC ubiquitination.
(J) In the presence of endogenous MEK1, Y177F mutant JmjC is not ubiquitinated compared with wild-type JmjC.