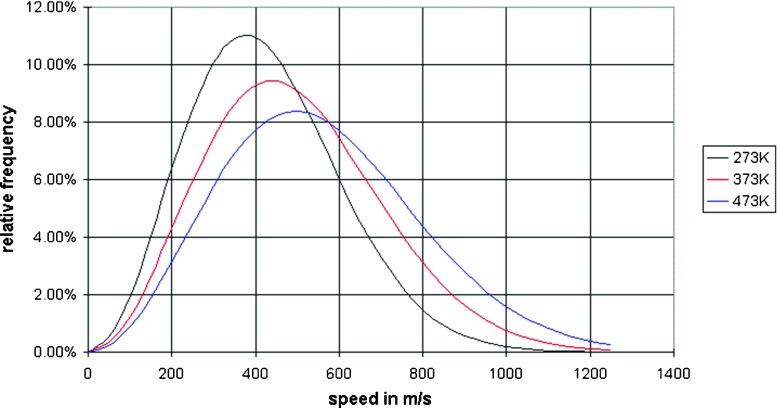
Fig. 3.
Maxwell–Boltzmann speed distributions at different temperatures. As the temperature increases, the curve will spread to the right and the value of the most probable kinetic energy will decrease. At temperature increases the probability of finding molecules at higher energy increases. Note also that the area under the curve is constant since total probability must be one