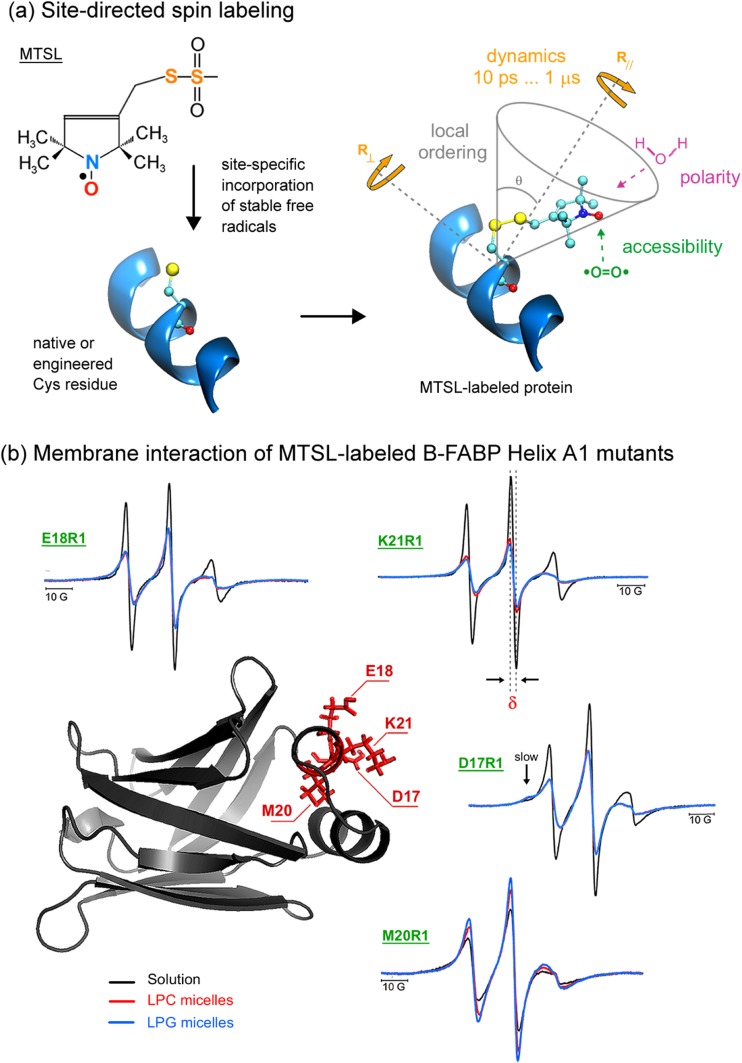
Fig. 2.
Spin labeling ESR from the protein perspective. a Attachment of methanethiosulfonate spin label (MTSL) to a native or an engineered cysteine residue give rise to the side chain designated as R1 of the spin-labeled protein. ESR can provide valuable local information of the probe vicinity. See text for more details. b Sites of introduction of single R1 residues, one at a time, in the α-helix A1 of the native structure of human B-FABP (PDB ID: 1JJX) along with the corresponding ESR spectra of the mutants D17R1, E18R1, M20R1, and K21R1 in the membrane-bound (lysophosphatidylcholine – LPC - red; lysophosphatidylglycerol – LPG - blue) and solution (black) states. The arrow in the D17R1 spectrum denotes the more immobilized, ordered spin population that appeared in the presence of the micelles. ESR spectra, acquired at room temperature and with a scan range of 100 G, were normalized to the number of spins to facilitate the analysis: the less intense the spectrum, the more broadened it is, which means a more packed or less mobile spin label. Arrows point to a second, more ordered component in the ESR spectra of D17R1 and G33R1. δ corresponds to the central linewidth, whose inverse value is proportional to the mobility. Adapted from Dyszy et al. (2013) with permission