Fig. 4.
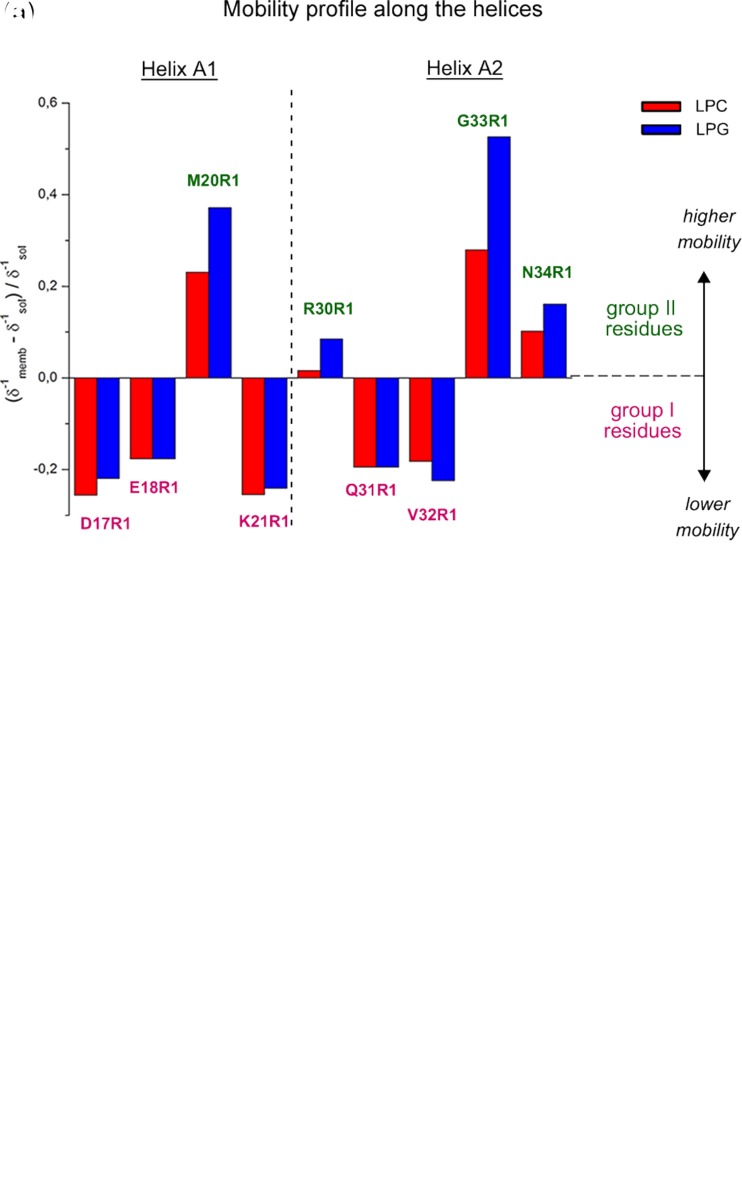
Putative structure–function–dynamics correlation of B-FABP. a Changes on the local mobility and polarity profiles of spin-labeled residues along helices A1 and A2 in the presence of LPC (red) or LPG (blue) micelles relative to the protein in solution. The mobility profile was calculated as the difference between the inverse central linewidth (δ−1) of the ESR spectrum of a particular residue in the membrane-bound state and the solution state and normalized to the δ−1 in the absence of the membrane. Positive values mean higher mobility of the MTSL probe relative to the membrane-unbound state, represented here by group II, green residues, whereas negative values indicate a more ordered state of the membrane-bound protein, represented by the group I, magenta residues. Note that only group II-residues experienced a significant lipid charge-dependent mobility changes, with the great disordering effect observed for G33R1. b Hypothetical membrane-docked B-FABP structure illustrating the two group of residues (group I, magenta; group II, green) that experience different conformational changes upon membrane interaction. Both mobility changes and structural rearrangements of helices A1 and A2 might contribute to membrane binding and FA delivery mechanisms. See text for more details. Adapted from (Dyszy et al. 2013) with permission