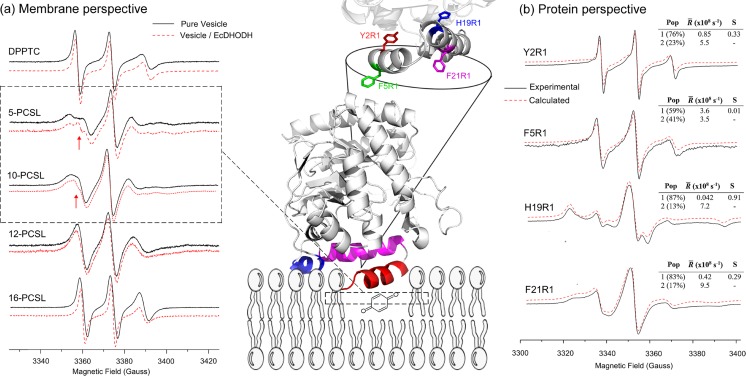
Fig. 5.
Unraveling EcDHODH function from protein and membrane perspectives. Center Cartoon representation of EcDHODH (PDB ID: 1F76) attached to a hypothetical bilayer membrane, fishing out the membrane-embedded quinone. The α-helix 1 (red) is responsible for membrane interaction, the α-helix 2 (magenta) and the 310 helix (blue) both act like a “rigid base” by which the α-helix 1 performs the open-to-close mechanism (see the text for more details). At the top is shown the protein amino acid positions used for SDSL (Y2 and F5 from α-helix 1; H19 and F21 from α-helix 2). a Membrane perspective ESR spectra of lipids labeled in the head group (DPPTC) and at positions 5, 10, 12, and 16 (n-PCSL) of the lipid acyl chain with (red) and without (black) EcDHODH. The narrower component of the two-spectral feature of 5- and 10-PCSL ESR spectra (arrows in the dashed square) reports on the defect-like structure induced by EcDHODH in the lipid bilayer. b Protein perspective Experimental (solid lines, black) and calculated (dashed lines, red) ESR signals of the single-cysteine EcDHODH mutants labeled with the MTSL probe along with the best-fit rotational diffusion rates (R) and order parameters (S) obtained from NLLS simulations. The relative population of the two components is also shown. Adapted from (Couto et al. 2008, 2011) with permission