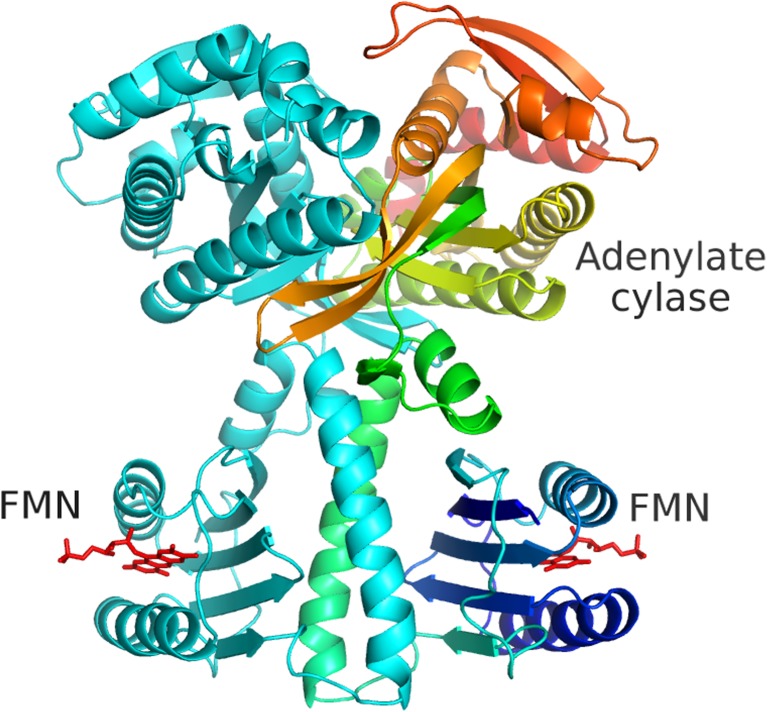
Fig. 5.
Ribbon diagram of the complete OaPAC dimer. Each subunit contains 366 residues, consisting of a BLUF domain, helical region (coiled-coil) and C-terminal adenylate cyclase domain. One dimer is coloured light blue, and the other is shown in dark blue (N-terminus) to red (C-terminus). The flavin molecules are shown as red stick models. Activation of the adenylate cyclase domains by light involves signal transduction from the flavin through the central coiled-coil to the active sites