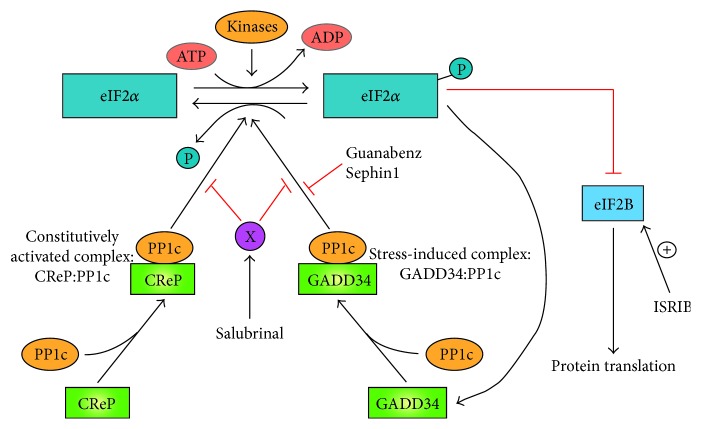
Figure 2.
Pharmacological regulation of the integrated stress response (ISR). The translation initiation factor eIF2α is phosphorylated (eIF2α-P) by a diverse group of kinases. Phosphorylated eIF2α represses eIF2B inducing translation attenuation as well as activation of a pathway that induces GADD34 forming a complex with PP1c (GADD34:PP1c). This stress-activated complex induces the desphosphorylation of eIF2α-P to restore proteostasis. A constitutively activated complex (CReP:PP1c) dephosphorylates eIF2α-P under basal conditions. Salubrinal inhibits indirectly the activity of both GADD34:PP1c and CReP:PP1c complexes through an unknown target (X). However, Guanabenz and Sephin1 only inhibit the activity of GADD34:PP1c complex. ISRIB overcomes the attenuation of translation induced by eIF2α-P, activating eIF2B downstream of eIF2α. ADP, adenosine diphosphate; ATP, adenosine triphosphate; CReP, constitutive repressor of eIF2α phosphorylation; eIF2α, eukaryotic translation initiation factor 2α; eIF2αB, eukaryotic translation initiation factor 2B; GADD34, growth arrest and DNA-damage inducible 34; ISRIB, integrated stress response inhibitor; P, inorganic phosphate; PP1c, protein phosphatase 1 catalytic subunit.