Abstract
A 78-year-old male who had received a dexamethasone implant (Ozurdex, Allergan, Inc., Irvine, CA, USA) 15 days back for recalcitrant diabetic macular edema in the left eye came to us for a second opinion. On examination, his corrected distance visual acuity was 20/20 in the right eye and 20/40 in the left eye. Early cataractous changes were present in both eyes. The intraocular pressure was within normal limits. The Ozurdex implant was seen lodged in the posterior cortex of the crystalline lens in the left eye, confirmed on anterior segment optical coherence tomography (OCT) and ultrasound biomicroscopy. Fundus examination showed moderate nonproliferative diabetic retinopathy in both eyes with macular edema and epiretinal membrane in the left eye, confirmed on OCT. The patient was noncompliant and returned after 10 months. Interestingly, the implant was still present in the same location with the same vision and anterior segment findings as before. The OCT showed a reduction in macular edema. The patient was advised regular follow-up and cataract surgery at a later date.
Key words: Cataract, diabetic macular edema, intralenticular Ozurdex
Ozurdex (DEX Implant; Allergan, Inc., Irvine, CA, USA) is a water-soluble biodegradable copolymer of lactic acid and glycolic acid containing micronized dexamethasone. It has been approved by the US Food and Drug Administration for the treatment of macular edema due to retinal vein occlusion. Several studies have confirmed its efficacy in diabetic macular edema as well.[1] It is a rod-shaped (6 mm) implant with 0.7 mg of dexamethasone releasing 100–1000 μg/ml of the drug per day for the first 2 months. Concentration of the drug becomes undetectable after 7–8 months.[2] Known complications of the implant include endophthalmitis, glaucoma, and cataract. Lenticular injury during Ozurdex implantation is rare but known.[3] To our knowledge, this report provides the longest follow-up of a case of intralenticular Ozurdex without cataract progression.
Case Report
A 78-year-old male with a history of Ozurdex implantation 2 weeks prior for resistant diabetic macular edema came to us for a second opinion. His corrected distance visual acuity (CDVA) was 20/20 in the right eye and 20/40 in the left eye. The anterior segment examination showed nuclear sclerosis grade 1 in both eyes. The Ozurdex implant was seen lodged inside the posterior cortex of the lens in the left eye. Fundus examination showed moderate nonproliferative diabetic retinopathy in both eyes with macular edema in the left eye. Optical coherence tomography (OCT) confirmed cystoid macular edema with central subfield macular thickness (CST) of 456 μm in the left eye [Fig. 1]. One end of the pellet was seen within the lens, but the rest was not visualized because of limited pupillary dilatation (4–5 mm). Anterior segment OCT and ultrasound biomicroscopy confirmed the location of the other end of Ozurdex to just anterior to the posterior capsule [Figs. 2 and 3].
Figure 1.
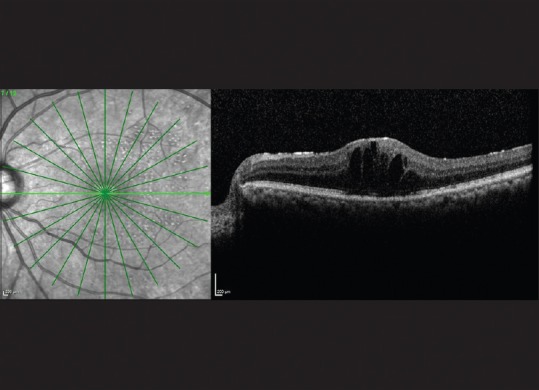
Optical coherence tomography of left eye showing patchy epiretinal membrane with cystoid macular edema (2 weeks after injection)
Figure 2.

Anterior segment optical coherence tomography of left eye showing intralenticular location of Ozurdex implant (arrow)
Figure 3.
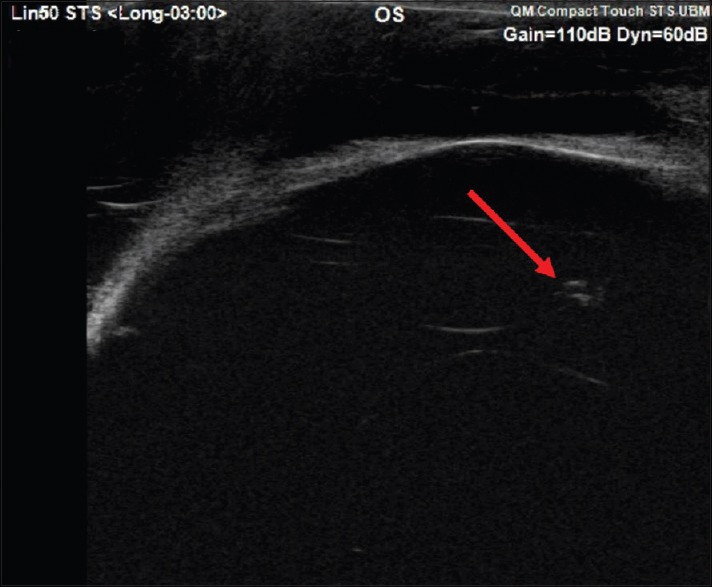
Ultrasound biomicroscopy of the left eye showing location of Ozurdex (arrow) pellet inside the lens
Since the intralenticular location of the Ozurdex implant was not significantly affecting the vision, intraocular pressure or anterior segment, only close follow-up with no immediate surgical intervention was planned. However, the patient was noncompliant and was lost to follow-up. He came back after 10 months with the implant still in situ [Fig. 4]. The CDVA was 20/40 in the left eye with no worsening of cataractous changes. A reduction in macular edema was noted and confirmed on OCT, showing a CST of 344 μm [Fig. 5].
Figure 4.
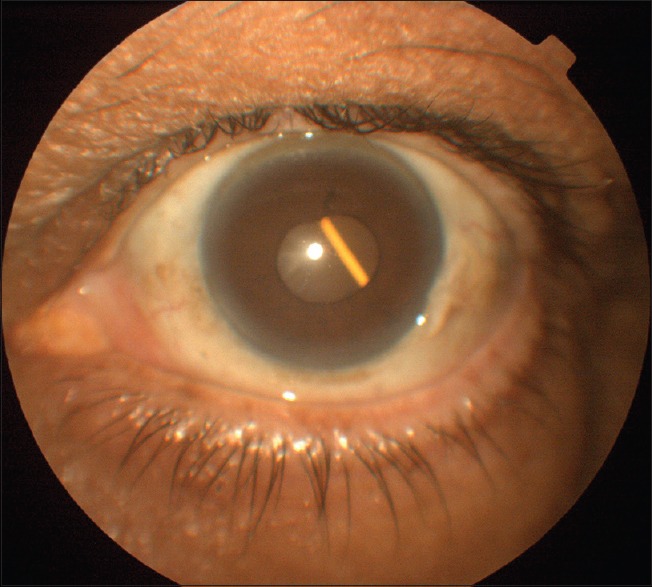
Slit-lamp photograph of the left eye showing Ozurdex pellet inside lens
Figure 5.
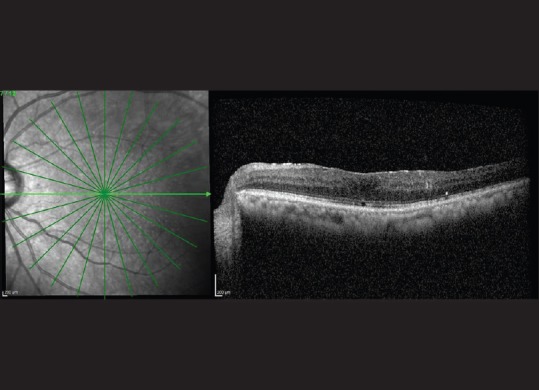
Optical coherence tomography of the left eye showing reduction in macular edema (10 months after injection)
Discussion
Lenticular injuries during intravitreal injections were found to be 0.009% in a multicentric case series study.[4] Ozurdex implantation is different from routine intravitreal injections because of the larger (22-gauge) needle used and the speed (0.8 m/s in vitreous),[5] by which the pellet is introduced into the vitreous cavity. Improper technique, an inexperienced surgeon, and patient head movement can all lead to inadvertent injection of Ozurdex implant into the lens.
Trauma to the lens with or without the presence of a foreign body can lead to cataractous changes. There have been reports of intralenticular Ozurdex leading to cataract progression in days or months after injection and rise in intraocular pressure requiring intervention.[3,6,7] Progression of cataract might take as long as 11 months after injection and might require a second Ozurdex injection for macular edema in the presence of intralenticular Ozurdex.[2] In our case, there was no progression of cataract and intraocular pressure was normal during the entire follow-up. The Ozurdex end inside the lens could have behaved like a sterile foreign body and did not cause any cataract or the site of Ozurdex entry might be through the equator of lens preserving posterior capsule could be the reasons for nonprogression of cataract in our case.
Coca-Robinot et al. and Sekeroglu et al. observed the therapeutic effect of intralenticular Ozurdex as long as 6 months in their case reports. Later, there was a progression of cataract requiring intervention.[8,9] In our patient, macular edema did not recur in the presence of the intralenticular Ozurdex. This could be explained by the fact that a part of implant which was in contact with the vitreous might have released small quantities of the drug sufficient enough to reduce macular edema, and in later phase, vitreous might have plugged the posterior end thus preventing progression of cataract.
Conclusion
Accidental intralenticular entry of an Ozurdex implant does not always necessitate immediate surgical removal. The patient can be followed up and a repeat implant needs to be reconsidered for persistent or recurrent cases of macular edema. Close follow-up for the development of cataract and the primary pathology is crucial.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Boyer DS, Yoon YH, Belfort R, Jr, Bandello F, Maturi RK, Augustin AJ, et al. Three-year, randomized, sham-controlled trial of dexamethasone intravitreal implant in patients with diabetic macular edema. Ophthalmology. 2014;121:1904–14. doi: 10.1016/j.ophtha.2014.04.024. [DOI] [PubMed] [Google Scholar]
- 2.Chang-Lin JE, Attar M, Acheampong AA, Robinson MR, Whitcup SM, Kuppermann BD, et al. Pharmacokinetics and pharmacodynamics of a sustained-release dexamethasone intravitreal implant. Invest Ophthalmol Vis Sci. 2011;52:80–6. doi: 10.1167/iovs.10-5285. [DOI] [PubMed] [Google Scholar]
- 3.Fasce F, Battaglia Parodi M, Knutsson KA, Spinelli A, Mauceri P, Bolognesi G, et al. Accidental injection of dexamethasone intravitreal implant in the crystalline lens. Acta Ophthalmol. 2014;92:e330–1. doi: 10.1111/aos.12332. [DOI] [PubMed] [Google Scholar]
- 4.Meyer CH, Rodrigues EB, Michels S, Mennel S, Schmidt JC, Helb HM, et al. Incidence of damage to the crystalline lens during intravitreal injections. J Ocul Pharmacol Ther. 2010;26:491–5. doi: 10.1089/jop.2010.0045. [DOI] [PubMed] [Google Scholar]
- 5.Meyer CH, Klein A, Alten F, Liu Z, Stanzel BV, Helb HM, et al. Release and velocity of micronized dexamethasone implants with an intravitreal drug delivery system: Kinematic analysis with a high-speed camera. Retina. 2012;32:2133–40. doi: 10.1097/IAE.0b013e31825699e5. [DOI] [PubMed] [Google Scholar]
- 6.Berarducci A, Sian IS, Ling R. Inadvertent dexamethasone implant injection into the lens body management. Eur J Ophthalmol. 2014;24:620–2. doi: 10.5301/ejo.5000436. [DOI] [PubMed] [Google Scholar]
- 7.Chhabra R, Kopsidas K, Mahmood S. Accidental insertion of dexamethasone implant into the crystalline lens-12 months follow-up. Eye (Lond) 2014;28:624–5. doi: 10.1038/eye.2014.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coca-Robinot J, Casco-Silva B, Armadá-Maresca F, García-Martínez J. Accidental injections of dexamethasone intravitreal implant (Ozurdex) into the crystalline lens. Eur J Ophthalmol. 2014;24:633–6. doi: 10.5301/ejo.5000439. [DOI] [PubMed] [Google Scholar]
- 9.Sekeroglu MA, Anayol MA, Koc F, Tirhis H, Ozkan SS, Yilmazbas P. Intralenticular sustained-release dexamethasone implant: Is it still effective on macular edema? Case Rep Ophthalmol. 2016;7:85–9. doi: 10.1159/000444163. [DOI] [PMC free article] [PubMed] [Google Scholar]


