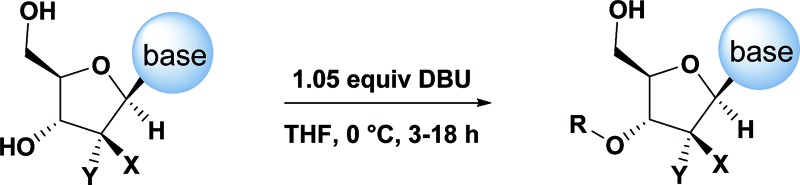
Table 2. Scope of the 3′-functionalization of nucleosides.
| |||||
| Entry | Nucleoside | Electrophile | Product | Yield e | 3′ : 5′ g |
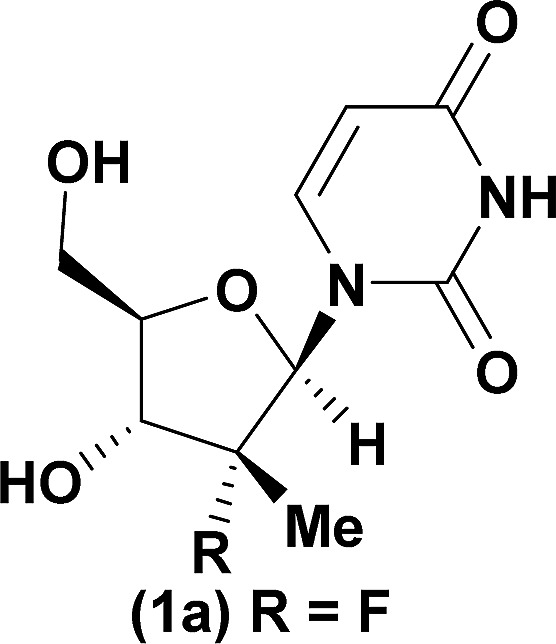
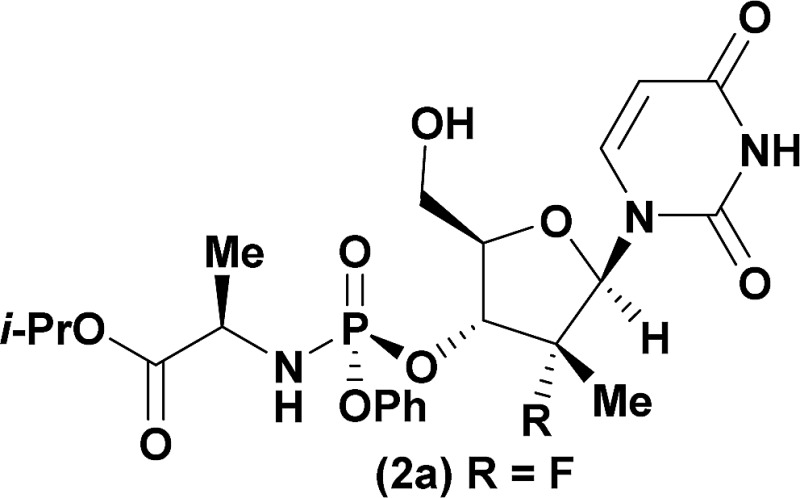
| 1 a |
|
|
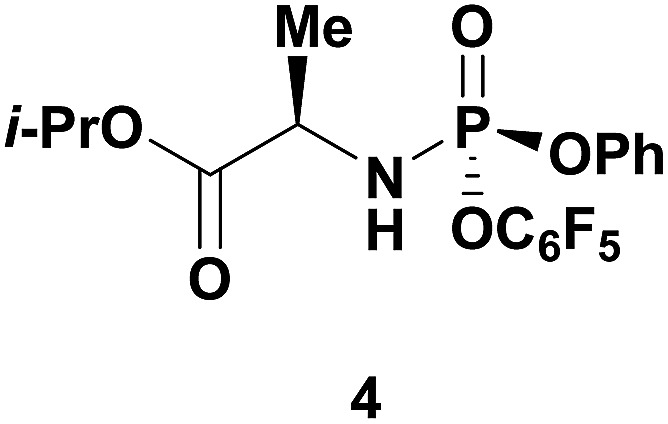
|
92 | 98 : 2 |
| 2 a | (1b) R = Cl | 4 | (2b) R = Cl | 89 | 98 : 2 |
| 3 a | (1c) R = –CCH | 4 | (2c) R = –CCH | 74 | 92 : 8 |
| 4 a | (1d) R = N3 | 4 | (2d) R = N3 | 62 | 91 : 9 |
| 5 a | (1e) R = CN | 4 | (2e) R = CN | 58 | 97 : 3 |
| 6 |
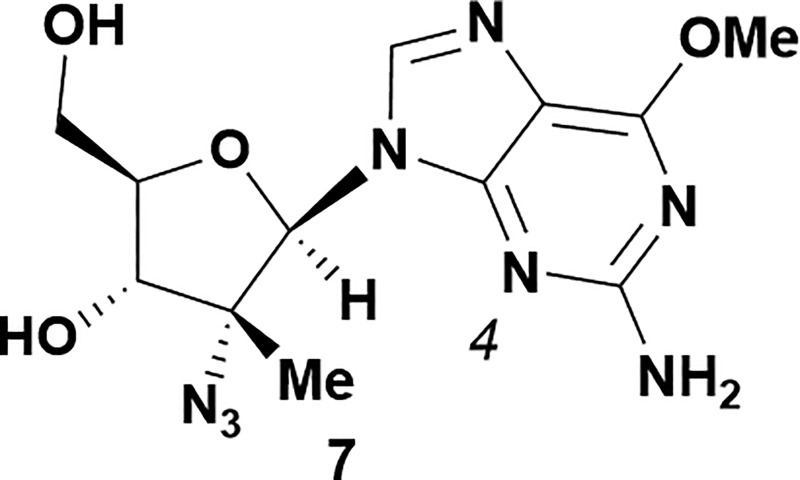
|
4 |
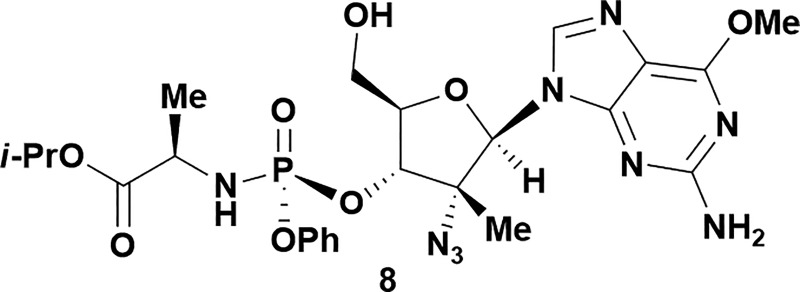
|
84 | ND |
| 7 |
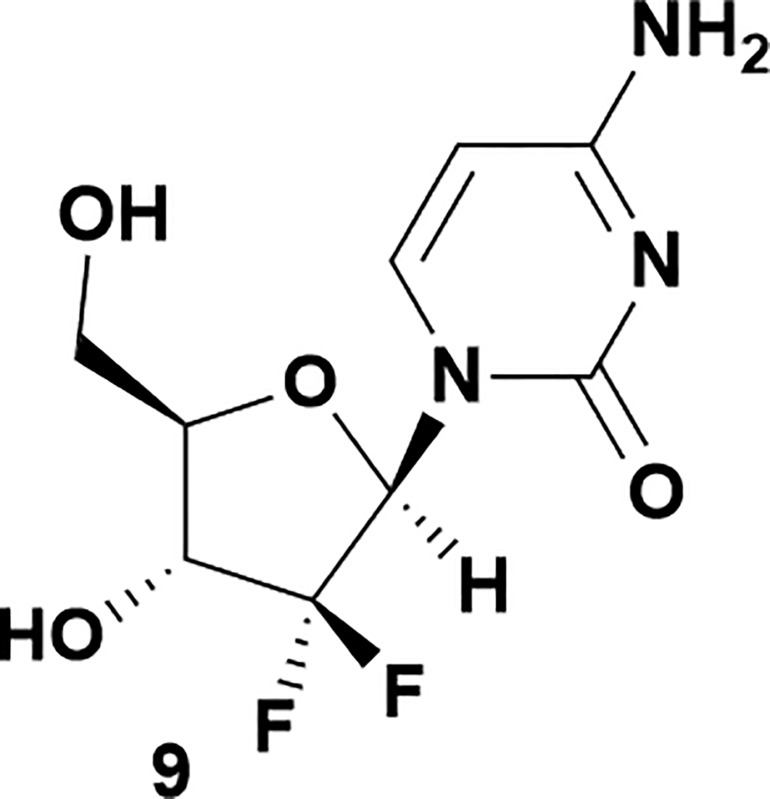
|
4 |
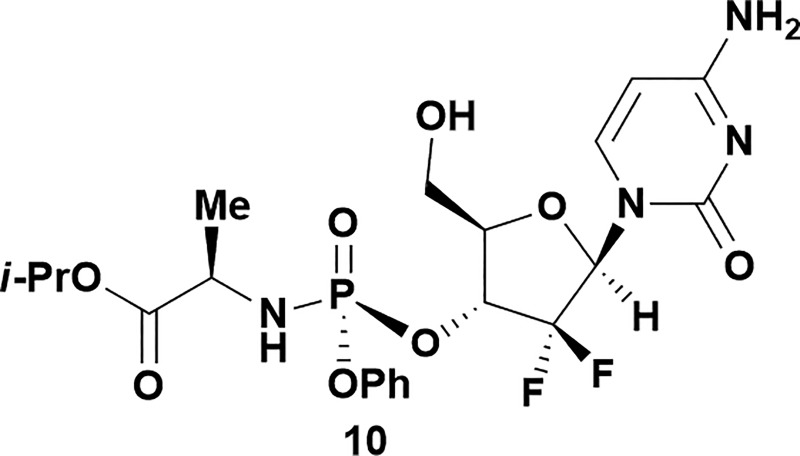
|
85 f | ND |
| 8 a , b |
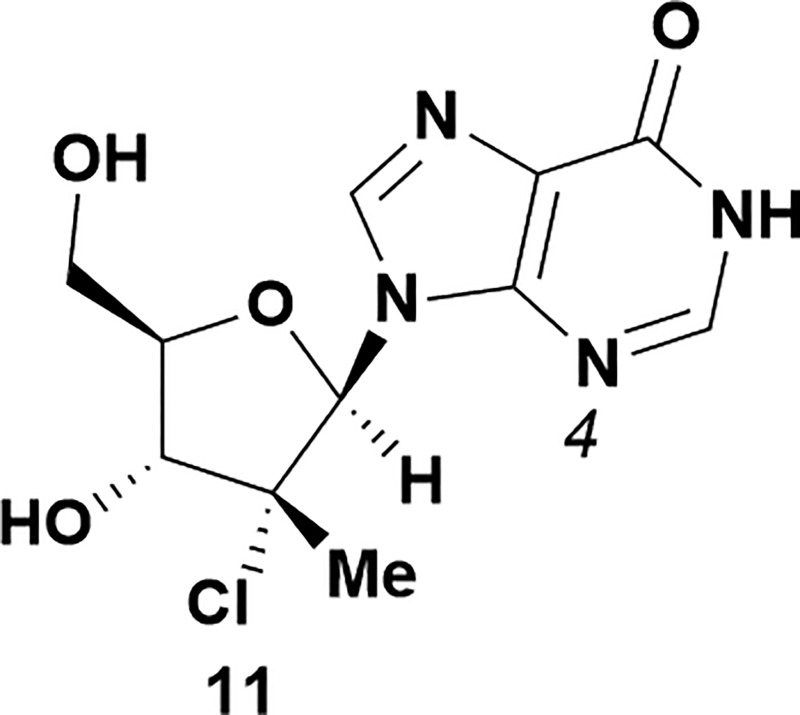
|
4 |
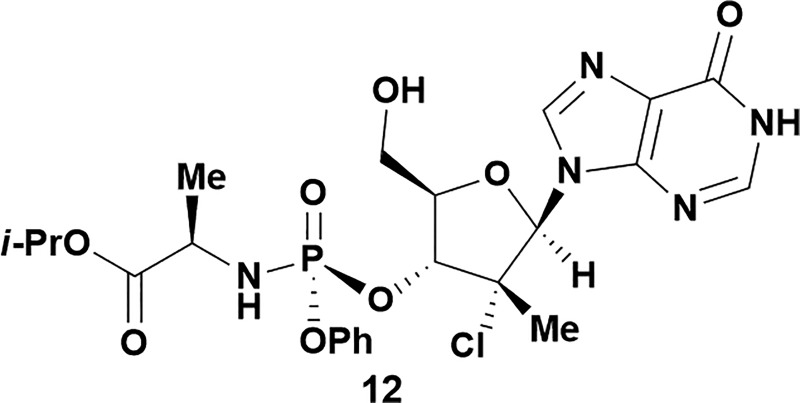
|
57 | 93 : 7 |
| 9 | 5 |
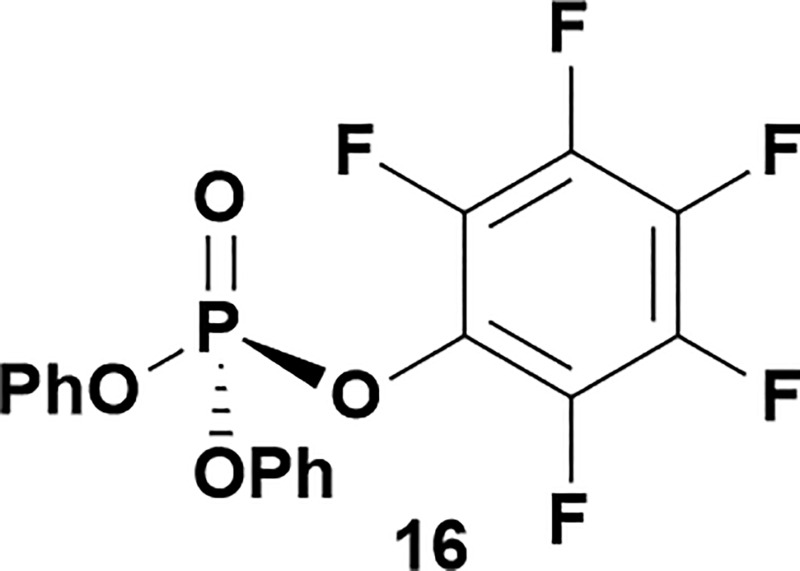
|
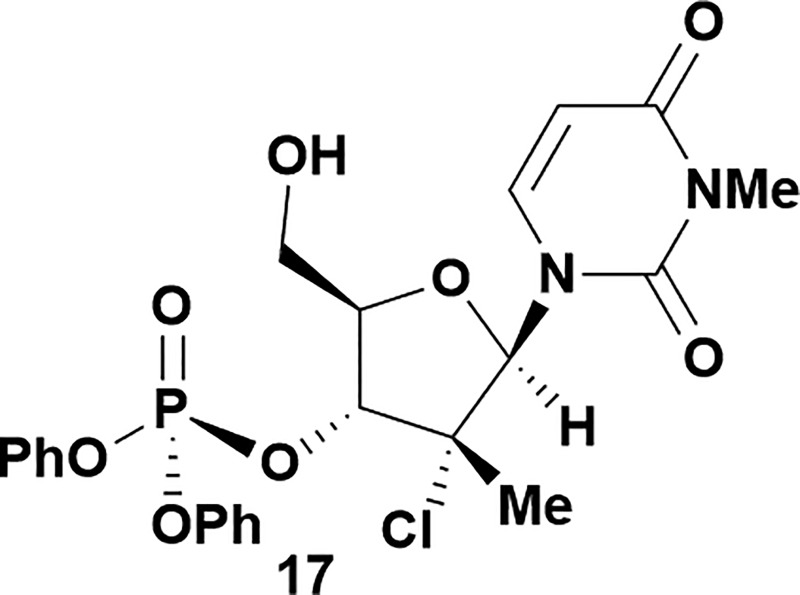
|
71 | 96 : 4 |
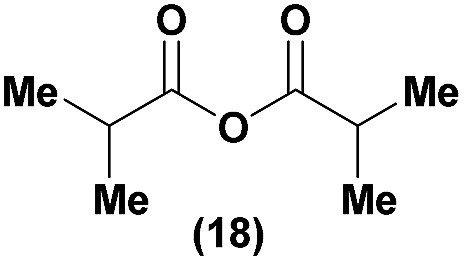
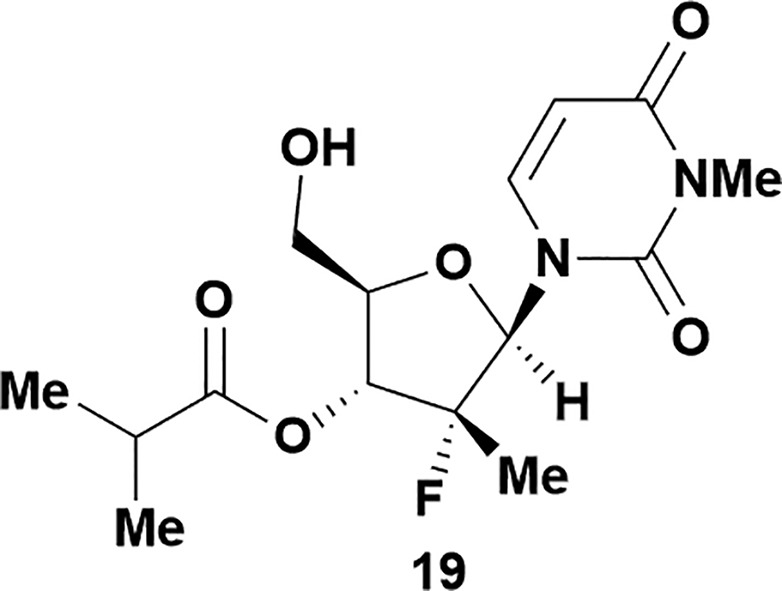
| 10 c | 5 |
|
|
59 (68 brsm) d | 99 : 1 |
aAnions are expected to be formed and serve as the structures modelled in the conformational analysis.
b2 equivalents of DBU used and temperature lowered to –15 °C.
cTemperature lowered to –15 °C.
dBased on recovered starting material (brsm).
eIsolated yields of pure 3′-phosphorated product and major p-epimer.
fAssay yield.
g3′ : 5′ selectivity determined by HPLC or UPLC, if labelled ND we were unable to resolve or detect the 5′-product the peaks by LC.
