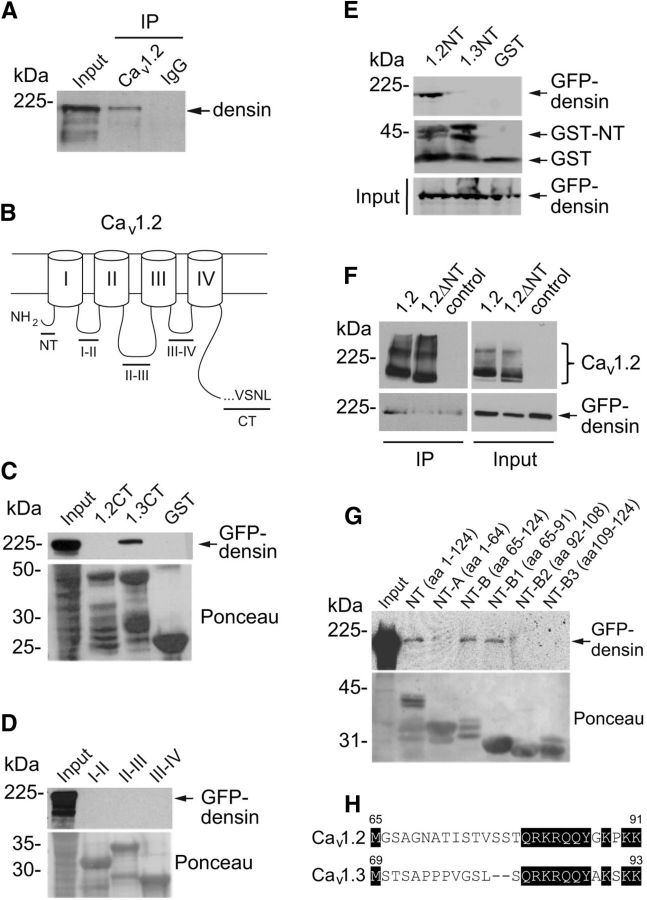
Figure 1.
Densin interacts with Cav1.2 channels in the brain and binds to a site in the NT domain of Cav1.2. A, Coimmunoprecipitation of densin with Cav1.2 antibodies but not rabbit control IgG in lysates of mouse prefrontal cortex. Western blotting was performed using a densin antibody. B, Schematic of Cav1.2 α1 subunit showing the NT domain, the C-terminal (CT) domain, and cytoplasmic loops (I–II, II–III, and III–IV). C–E, Pull-down assays using GST-tagged cytoplasmic domains of Cav1.2. Input represents GFP-tagged densin in lysates of transfected HEK293T cells. C, Densin binds to the CT of Cav1.3, but not that of Cav1.2. D, Densin does not bind to the cytoplasmic loops of Cav1.2. E, Densin binds to the NT of Cav1.2, but not that of Cav1.3. F, Coimmunoprecipitation of GFP-densin with FLAG antibodies in HEK293T cells cotransfected with FLAG-tagged Cav1.2 (1.2), but not with Cav1.2 lacking the NT (1.2ΔNT) or control cells transfected with GFP-densin alone. G, Same as in C–E except with GST-tagged fragments of the Cav1.2 NT. Amino acid numbers are indicated in parentheses. All blots shown are representative of at least three independent experiments. Input lanes represent 7–10% of starting material. H, Sequence alignment of the B1 regions of Cav1.2 and Cav1.3. Conserved residues are shaded in black. Numbers indicate the amino acid residues.