Figure 1.
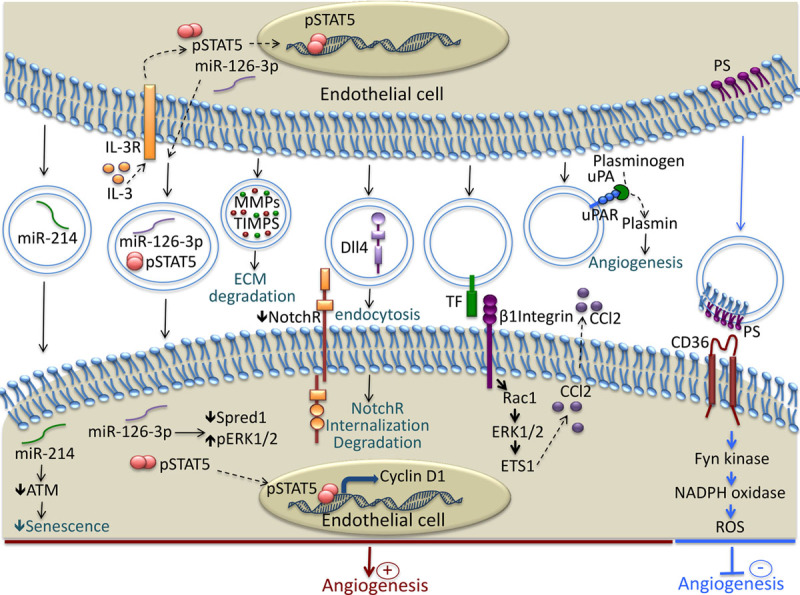
Mechanisms involved in the modulation of Angiogenesis by endothelial cell (EC)–derived extracellular vesicles (EVs). EC release EVs rich in micro-RNA such as miR-214 and miR-126, that are transferred to recipient EC and induce proangiogenic signaling. EVs contain functional matrix metalloproteinases that facilitate angiogenesis through the degradation of components of the extracellular matrix. Dll4 is transferred to EC by the EVs and induces Notch receptor internalization and tip cell formation. EVs bear, at their surface, a tissue factor that interacts with β1 integrin and induces Rac1-ERK1/2-ETS1 signaling, leading to the increased secretion of CCL2. EVs transport the complex uPA/uPAR, which stimulates angiogenesis through plasmin generation. The phosphatidylserine present on the surface of the EVs interacts with CD36 and induces Fyn kinase signaling, which leads to increased oxidative stress and the inhibition of angiogenesis. ATM indicates ataxia telangiectasia mutated; CCl2, chemokine c-c motif ligand 2; Dll4, Delta-like 4; ECM, extracellular matrix; ERK1/2, extracellular signal-related kinase 1 and 2; ETS1, avian erythroblastosis virus E26 homolog-1; IL-3R, interleukin-3 receptor; MMPs, matrix metalloproteinases; NotchR, Notch receptor; PS, phosphatidylserine; Rac1, Ras-related C3 botulinum toxin substrate 1; ROS, reactive oxygen species; TF, tissue factor; TIMPS, tissue inhibitor of metalloproteinases; uPA, urokinase plasminogen activator; and uPAR, urokinase plasminogen activator receptor.
