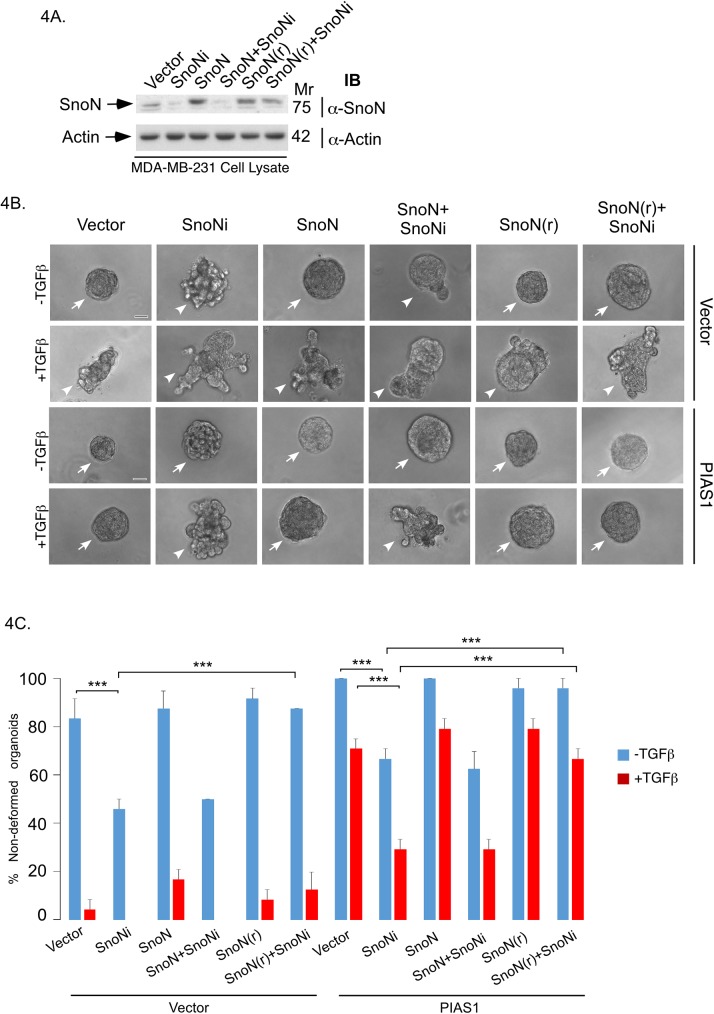
Fig 4. Endogenous SnoN mediates the ability of PIAS1 to suppress the invasive growth of breast cancer cell-derived organoids.
(A) SnoN and actin immunoblots of lysate of MDA-MB-231 cells cotransfected with a plasmid encoding a short hairpin RNA targeting a specific region of SnoN mRNA or a control plasmid, together with an expression plasmid encoding an RNAi-resistant SnoN (SnoN(r)) or wild type RNAi-sensitive SnoN, or the corresponding vector control. (B) Representative DIC light microscopy micrographs of untreated or 100pM TGFβ-treated 8-day old three-dimensional organoids derived from MDA-MB-231 cells stably expressing PIAS1 or the corresponding vector control, and co-transfected with SnoN RNAi, SnoN(r), wild type SnoN, or respective control plasmids. (C) Bar graph depicts mean ± SEM proportion of non-deformed organoids expressed as a percentage of total colonies counted for each experimental condition from three independent experiments including the one shown in B. Non-deformed organoids represents non-invasive growth phenotype. Knockdown of endogenous SnoN promoted invasive growth of organoids as compared to vector control or PIAS1 stably expressing cells in the absence or presence of TGFβ. Expression of SnoN(r) but not wild type SnoN suppressed the ability of SnoN RNAi to promote invasive growth of organoid in vector control or PIAS1 expressing cells in the absence or presence of TGFβ. Significant difference, ANOVA: ***P≤0.001. Scale bar indicates 50 μm. Arrows and arrowheads indicate non-deformed and invasive organoids, respectively.