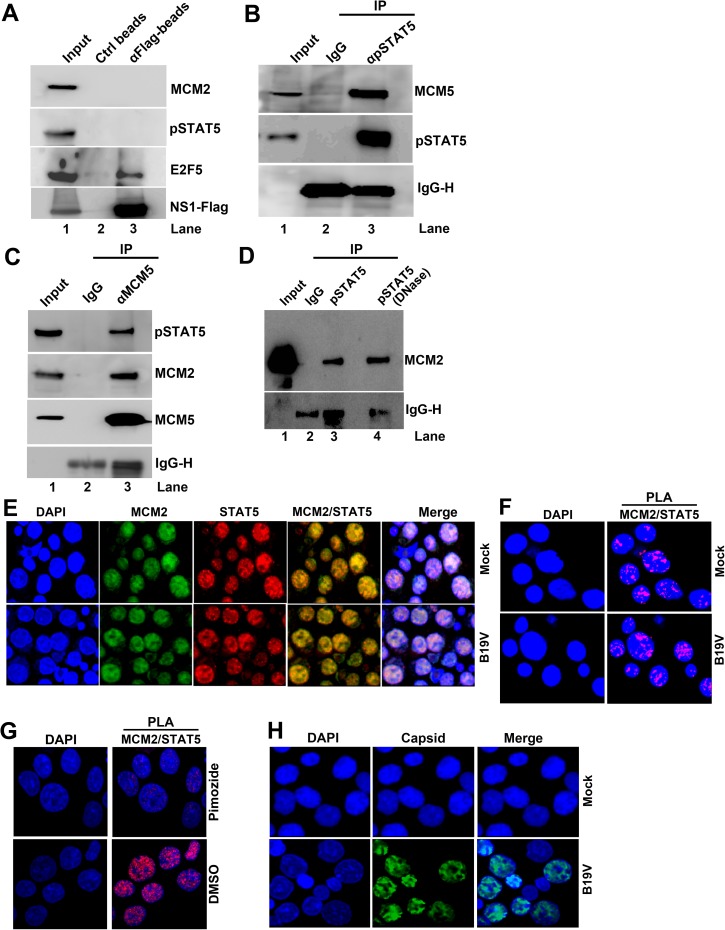
Fig 7. pSTAT5, but not NS1, interacts with the MCM complex.
(A) Immunoprecipitation (IP) assay. Cell lysates of NS1Flag-expressing UT7/Epo-S1 cells were prepared for pull-down assays with either anti-Flag-conjugated beads or control beads. Immunoprecipitated proteins were examined for the presence of MCM2 by Western blotting. Blots were reprobed with rabbit anti-pSTAT5(Y694), anti-E2F5, and anti-Flag antibodies. Detection of E2F5 was used as a positive control for NS1 IP. (B) Co-IP assay. UT7/Epo-S1 cells were collected, washed, and lysed with RIPA buffer. After centrifugation, the supernatant was incubated with either rabbit anti-pSTAT5(Y694) or control IgG antibody. Immunoprecipitated proteins were blotted for the presence of the MCM complex with an anti-MCM5 antibody and for pSTAT5 with rabbit anti-pSTAT5(Y694). (C) Reverse Co-IP assay. Reverse Co-IP was performed with an anti-MCM5 antibody. Immunoprecipitated proteins were examined for pSTAT5, MCM2, and MCM5, respectively. (D) Co-IP of lysates treated with DNase. UT7/Epo-S1 cell lysates, either treated or untreated with DNase (750 units of Benzonase) were incubated with anti-pSTAT5(Y694) or control IgG antibodies for Co-IP assay, and immunoprecipitated proteins were examined for MCM2 by Western blot analysis. (E-H) Immunofluorescence analysis. (E&F) Mock- or B19V-infected CD36+ EPCs were co-stained with rabbit anti-STAT5 and mouse anti-MCM2 antibodies, followed by (E) incubation with respective secondary antibodies, or by (F) proximal ligation assay, which produces amplified signal for labeled molecules in close proximity. (G) CD36+ EPCs were incubated with either DMSO or pimozide (at 30 μM) for 2 days. And then the cells were co-stained with rabbit anti-STAT5 and mouse anti-MCM2 antibodies for proximity ligation assay. (H) Infected EPCs were stained with an anti-capsid antibody. Confocal images were taken with an Eclipse C1 Plus (Nikon) microscope at 100 × magnification.