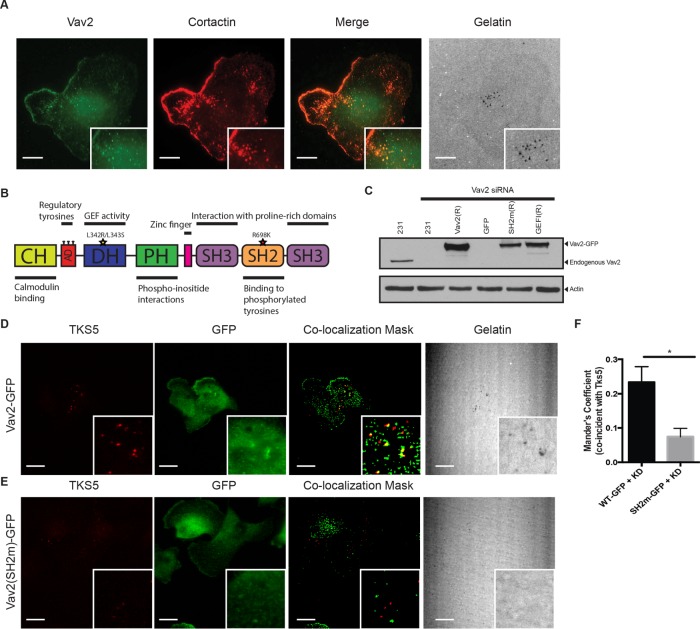
FIGURE 4:
Localization of Vav2 to invadopodia is mediated by its SH2 domain. (A) Colocalization of Vav2 and cortactin at sites of gelatin degradation. Insets, blow-ups of sites of invadopodia action. Scale bars, 10 μm. (B) Domain diagram of Vav2 indicating relevant mutations: SH2mut (R698K) and GEFi (L342R/L343S). (C) Western blot analysis of MDA-MB-231 cells transfected with control or Vav2 siRNA and cell lines stably expressing RNAi-resistant rescue constructs with wild-type Vav2-GFP (Vav2(R)), Vav2SH2m-GFP (SH2m(R)), or Vav2GEFi-GFP (GEFI(R)). Blots were immunoblotted for Vav2 and for β-actin as a loading control. (D–F) Endogenous Vav2 was knocked down in cells stably expressing RNAi-resistant Vav2-GFP (D) or Vav2SH2m-GFP (E). Cells were plated on fluorescent gelatin and immunostained for TKS5 and GFP. Scale bars, 10 μm. (F) Quantification of colocalization of WT and SH2m Vav2 with Tks5 at invadopodia.