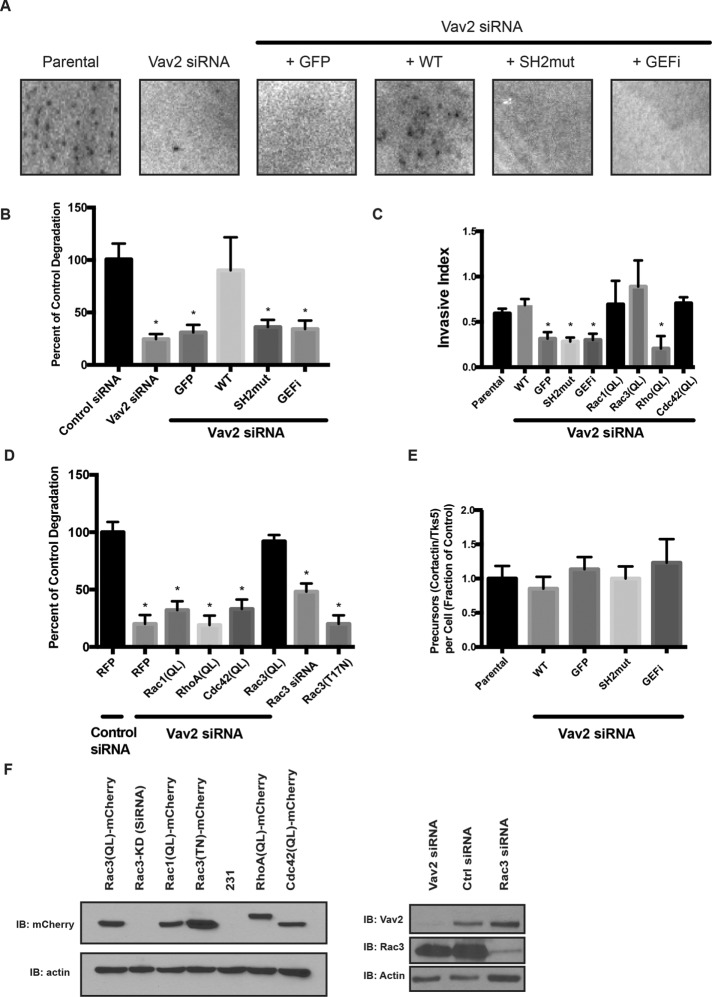
FIGURE 5:
Degradation of ECM requires both the SH2 domain and the GEF function of Vav2, and a constitutively active form of Rac3 can rescue Vav2 deficiency. Parental MDA-MB-231 cells or cells stably expressing GFP-tagged, RNAi-resistant WT or mutants of Vav2 were treated with control siRNA or Vav2 siRNA (Vav2 KD), plated on a fluorescent matrix, and allowed to degrade for 18 h. (A) Representative images of degradation area formed by the different cell lines. (B) Quantification of invadopodia matrix degradation area per cell. Ten fields were averaged in triplicate experiments. Vav2-knockdown and mutant constructs degrade significantly less matrix than wild-type constructs or control (p < 0.05). (C) Parental and mutant MDA-MB-231 cells were plated on a Matrigel-coated Transwell insert and allowed to migrate toward serum-containing medium. Relative invasion through Matrigel was quantified and normalized to proteolysis-independent migration. Vav2-knockdown and mutant constructs have a significant defect in Transwell invasion, but constitutively active Rac1 (Q61L), Rac3 (Q61L), RhoA (Q63L), or Cdc42 (Q61L; QL mutants) rescue the Vav2-knockdown phenotype (p < 0.05). Experiments performed in triplicate (n = 10 per condition). (D) Cells were treated with a control siRNA, Vav2 siRNA, or Rac3 siRNA or transfected with a dominant-negative (T17N) form of Rac3. Quantification of invadopodia matrix degradation area per cell for each of the cell types normalized to control. Ten fields were averaged in triplicate experiments. (E) Quantification of invadopodium formation (as measured by cortactin/Tks5 double-positive puncta). Cells were serum starved overnight in the presence of MMP inhibitor and treated with EGF (n = 20 per condition). (F) Western blot analysis of MDA-MB-231 cells transfected with control siRNA, Rac3 siRNA, and Vav2 siRNA and cell lines stably expressing rescue constructs with mCherry-tagged dominant-negative Rac3(TN), constitutively active Rac1(QL), Rac3(QL), RhoA(QL), or Cdc42(QL). Samples were immunoblotted for Rac3, Vav2, or mCherry and for β-actin as a loading control.