Abstract
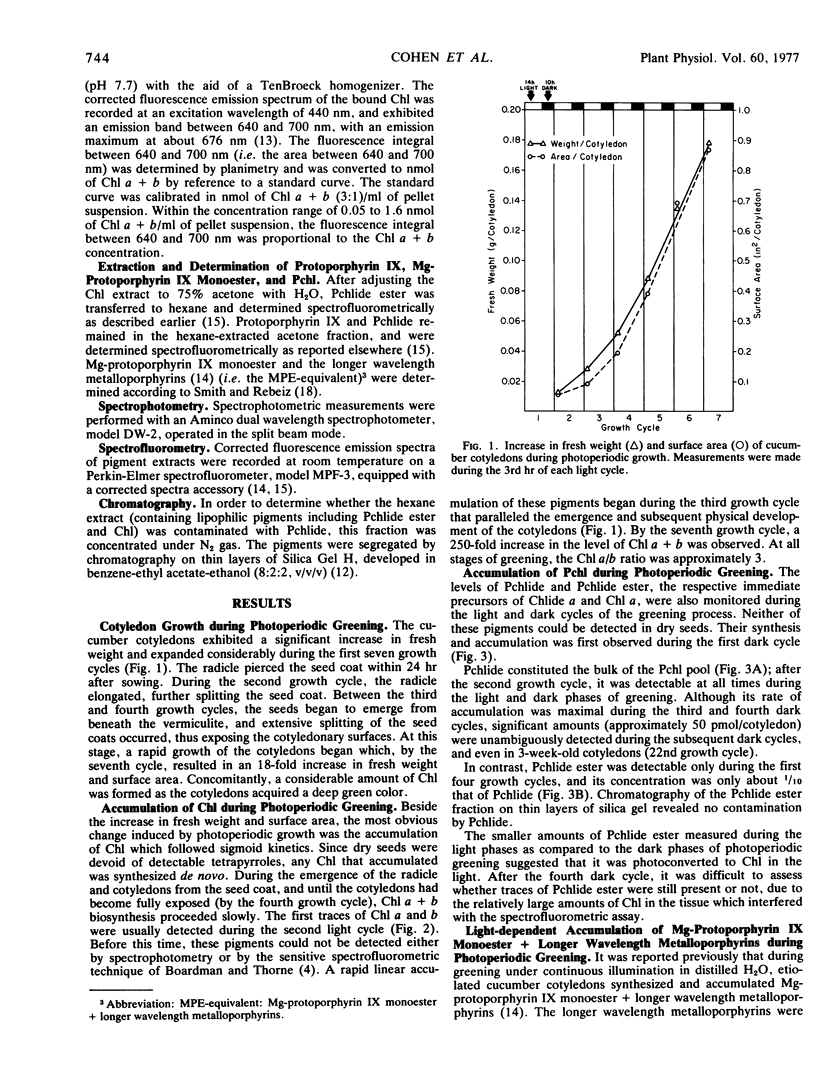
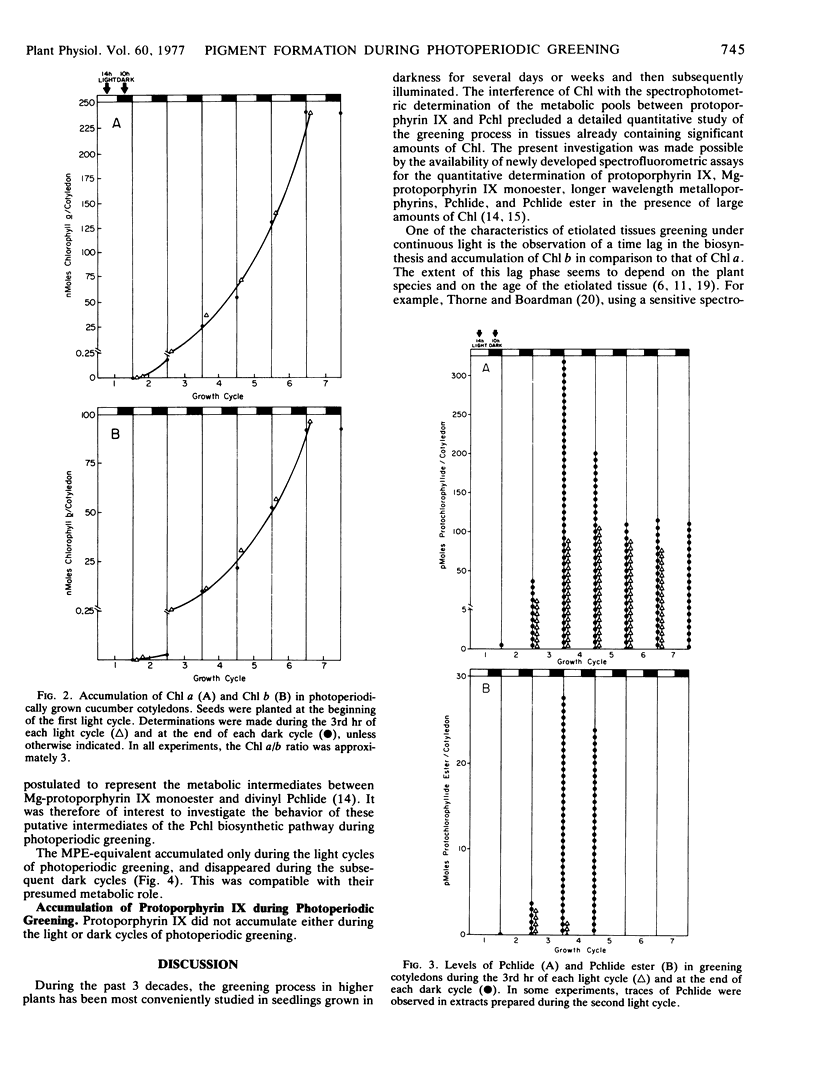
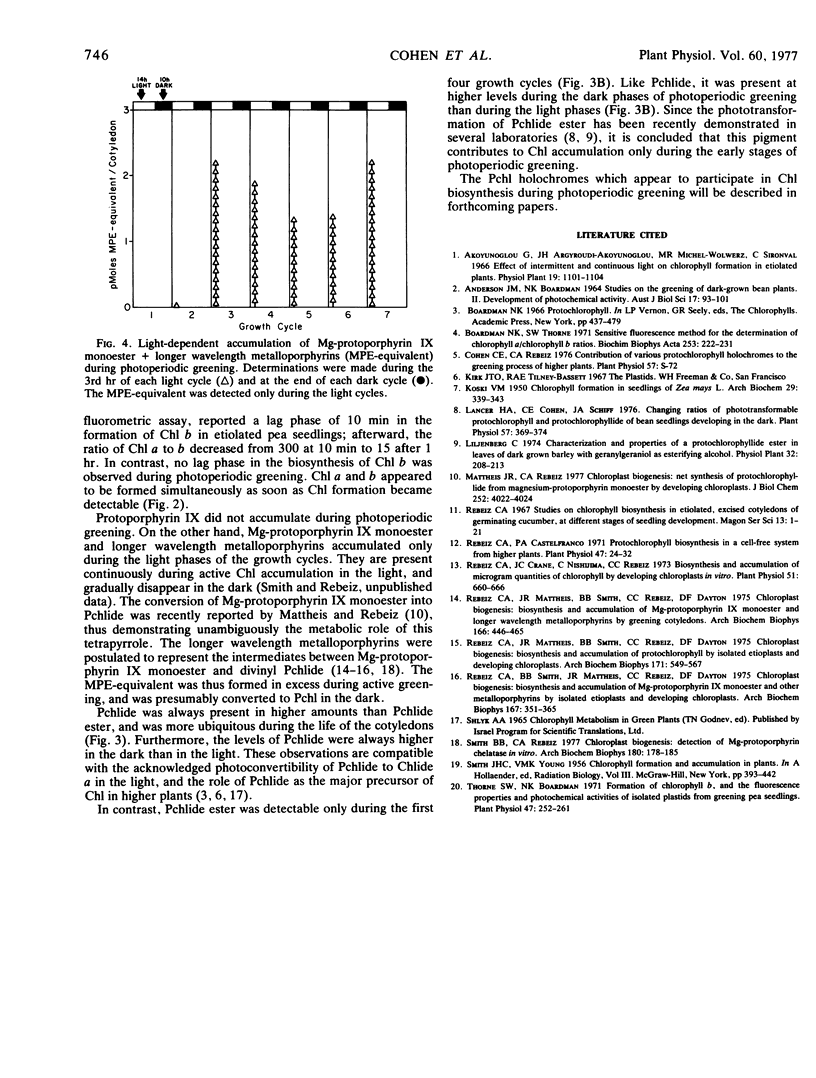
A study of greening in cucumber (Cucumis sativus L.) cotyledons grown under a light (14-hour) dark (10-hour) photoperiodic regime was undertaken. The pools of protoporphyrin IX, Mg-protoporphyrin IX monoester, protochlorophyllide, and protochlorophyllide ester were determined spectrofluorometrically. Chlorophyll a and b were monitored spectrophotometrically. Pigments were extracted during the 3rd hour of each light period and at the end of each subsequent dark period during the first seven growth cycles. Protoporphyrin IX did not accumulate during greening. Mg-protoporphyrin IX monoester and longer wavelength metalloporphyrins accumulated during the light cycles and disappeared in the dark. Their disappearance was accompanied by the accumulation of protochlorophyll. Higher levels of protochlorophyll were observed in the dark than in the light, and the greatest accumulation occurred during the third and fourth dark cycles. Protochlorophyllide was present in 3- to 10-fold excess over protochlorophyllide ester; it was detectable during the period of net chlorophyll accumulation as well as afterward. In contrast, protochlorophyllide ester was observable only during the first four photoperiodic cycles, suggesting that it was a metabolic intermediate only during the early stages of chlorophyll accumulation. Between the third and fourth growth cycles, a rapid increase in area and fresh weight per cotyledon began. This was accompanied by a 250-fold increase in the level of chlorophyll a + b during the three subsequent growth cycles. No lag period in the accumulation of chlorophyll b was observed, and at all stages of greening, the chlorophyll a/b ratio was approximately 3.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boardman N. K., Thorne S. W. Sensitive fluorescence method for the determination of chlorophyll a-chlorophyll b ratios. Biochim Biophys Acta. 1971 Nov 2;253(1):222–231. doi: 10.1016/0005-2728(71)90248-9. [DOI] [PubMed] [Google Scholar]
- Jackson A. O., Larkins B. A. Influence of Ionic Strength, pH, and Chelation of Divalent Metals on Isolation of Polyribosomes from Tobacco Leaves. Plant Physiol. 1976 Jan;57(1):5–10. doi: 10.1104/pp.57.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOSKI V. M. Chlorophyll formation in seedlings of Zea mays L. Arch Biochem. 1950 Dec;29(2):339–343. [PubMed] [Google Scholar]
- Lancer H. A., Cohen C. E., Schiff J. A. Changing ratios of phototransformable protochlorophyll and protochlorophyllide of bean seedlings developing in the dark. Plant Physiol. 1976 Mar;57(3):369–374. doi: 10.1104/pp.57.3.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattheis J. R., Rebeiz C. A. Chloroplast biogenesis. Net synthesis of protochlorophyllide from magnesium-protoporphyrin monoester by developing chloroplasts. J Biol Chem. 1977 Jun 25;252(12):4022–4024. [PubMed] [Google Scholar]
- Rebeiz C. A., Castelfranco P. A. Protochlorophyll biosynthesis in a cell-free system from higher plants. Plant Physiol. 1971 Jan;47(1):24–32. doi: 10.1104/pp.47.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebeiz C. A., Crane J. C., Nishijima C., Rebeiz C. C. Biosynthesis and accumulation of microgram quantities of chlorophyll by developing chloroplasts in vitro. Plant Physiol. 1973 Apr;51(4):660–666. doi: 10.1104/pp.51.4.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebeiz C. A., Mattheis J. R., Smith B. B., Rebeiz C. C., Dayton D. F. Chloroplast biogenesis. Biosynthesis and accumulation of protochlorophyll by isolated etioplasts and developing chloroplasts. Arch Biochem Biophys. 1975 Dec;171(2):549–567. doi: 10.1016/0003-9861(75)90065-x. [DOI] [PubMed] [Google Scholar]
- Rebeiz C. A., Mattheis J. R., Smith B. B., Rebeiz C., Dayton D. F. Chloroplast biogenesis. Biosynthesis and accumulation of Mg-protoprophyrin IX monoester and longer wavelength metalloporphyrins by greening cotyledons. Arch Biochem Biophys. 1975 Feb;166(2):446–465. doi: 10.1016/0003-9861(75)90408-7. [DOI] [PubMed] [Google Scholar]
- Rebeiz C. Z., Smith B. B., Mattheis J. R., Rebeiz C. C., Dayton D. F. Chloroplast biogenesis. Biosynthesis and accumulation of Mg-protoporphyrin IX monoester and other metalloporphyrins by isolated etioplasts and developing chloroplasts. Arch Biochem Biophys. 1975 Mar;167(1):351–365. doi: 10.1016/0003-9861(75)90471-3. [DOI] [PubMed] [Google Scholar]
- Smith B. B., Rebeiz C. A. Chloroplast biogenesis: detection of Mg-protoporphyrin chelatase in vitro. Arch Biochem Biophys. 1977 Apr 15;180(1):178–185. doi: 10.1016/0003-9861(77)90023-6. [DOI] [PubMed] [Google Scholar]
- Thorne S. W., Boardman N. K. Formation of chlorophyll B, and the fluorescence properties and photochemical activities of isolated plastids from greening pea seedlings. Plant Physiol. 1971 Feb;47(2):252–261. doi: 10.1104/pp.47.2.252. [DOI] [PMC free article] [PubMed] [Google Scholar]


