Figure 8.
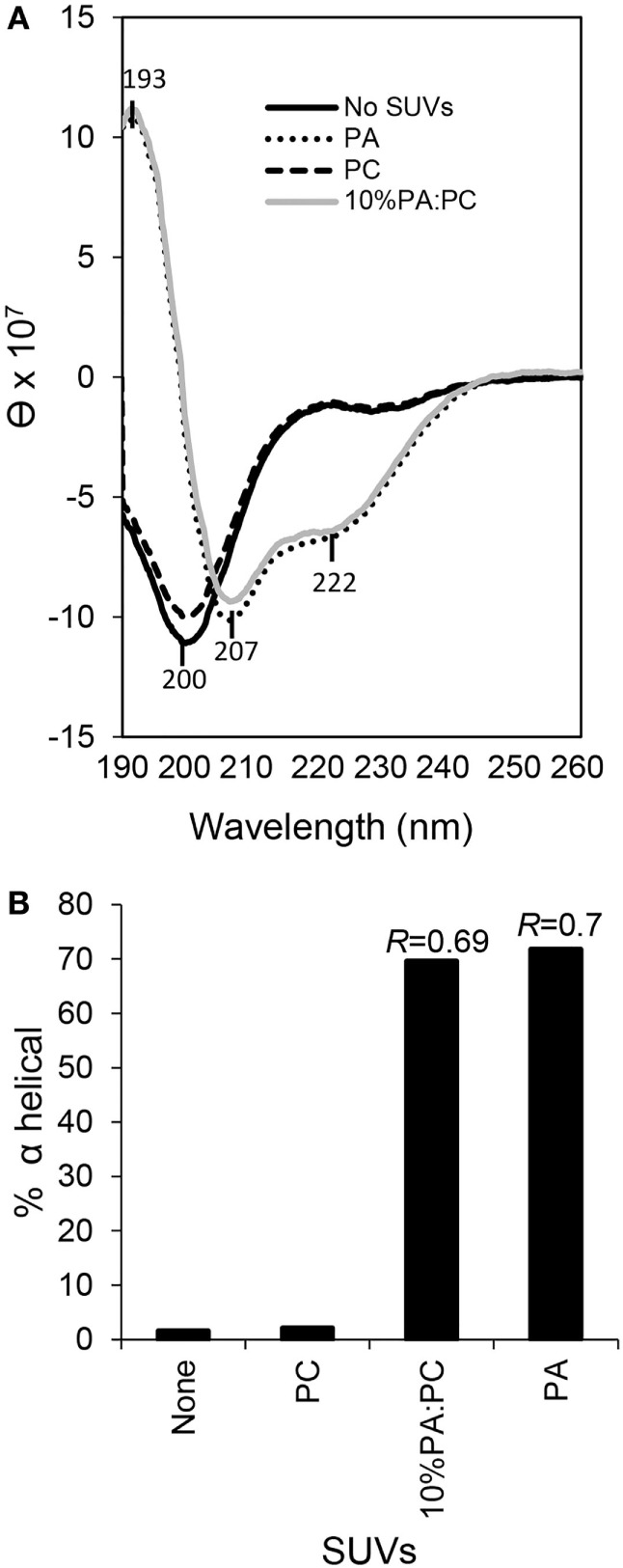
Secondary structure of recombinant SpTransformer protein, rSpTransformer-E1 (rSpTrf-E1), transforms from intrinsically disordered to α helical in the presence of phosphatidic acid (PA) small unilamellar vesicles (SUVs). (A) CD spectra show intrinsic disorder or random coils for 0.25 mM rSpTrf-E1 in 10 mM sodium phosphate buffer in the absence of PA or in the presence of 100% phosphatidylcholine SUVs (PC). rSpTrf-E1 transforms to α helical secondary structure in the presence of 10% PA:PC SUVs or 100% PA SUVs (PA). θ is the mean residue ellipticity with standard units of degrees × cm2 × dmol−1 as described (17). (B) The percentage of α helical structure for rSpTrf-E1 is 1.57% in the absence of lipids and 2.1% in the presence of PC. However, in the presence of 100% PA SUVs or 10% PA/PC SUVs, the α helical structure of rSpTrf-E1 is 69.6 and 71.8%, respectively. The percentage of secondary structure for rSpTrf-E1 in the presence of PA are based the deconvolution of the spectra data using the DichroWeb server. The R values [ellipticity ratios: R = θ222/θ207 shown in panel (A)] are indicated for the CD analysis of rSpTrf-E1 with 10% PA:PC and for 100% PA (17).
