Abstract
Objective
We examined the associations of maternal caffeine intake during pregnancy with offspring growth patterns, and body fat and insulin levels at school-age.
Methods
In a population-based birth cohort among 7,857 mothers and their children, we assessed maternal caffeine intake during pregnancy by questionnaires. Growth characteristics were measured from birth onwards. At 6 years, body fat and insulin levels were measured.
Results
Compared to children whose mothers consumed <2 units of caffeine per day during pregnancy (1 unit of caffeine is equivalent to 1 cup of coffee (90 mg caffeine)), those whose mothers consumed ≥6 units of caffeine per day tended to have a lower weight at birth, higher weight gain from birth to 6 years and higher body mass index from 6 months to 6 years. Both children whose mothers consumed 4-5.9 and ≥6 units of caffeine per day during pregnancy tended to have a higher childhood body mass index and total body fat mass. Only children whose mothers consumed ≥6 units of caffeine per day had a higher android/gynoid fat mass ratio.
Conclusions
Our results suggest that high levels of maternal caffeine intake during pregnancy are associated with adverse offspring growth patterns and childhood body fat distribution.
Keywords: Caffeine, pregnancy, growth, fat mass, insulin
Introduction
Caffeine is frequently consumed during pregnancy (1). Caffeine crosses the placenta and enters the fetal circulation freely (2). Fetal exposure to caffeine is prolonged as a result of a slow clearance of caffeine in pregnant women and slow fetal metabolism (3). We and other previous studies have reported associations of high levels of maternal caffeine intake during pregnancy with higher risks of low birth weight (4–7). High maternal caffeine intake during pregnancy was also associated with impaired fetal length growth from second trimester onwards (7).
Although previous studies consistently suggested that children born with a low birth weight are at higher risk of an adverse body fat distribution and insulin resistance in later life (8–12), not much is known about the direct long-term offspring consequences of maternal caffeine intake during pregnancy. A recent prospective cohort study in the United States among 615 mothers and children, reported a higher overall risk of obesity before the age of 15 years in children exposed to any caffeine during pregnancy (13). Another recent study among 1986 mothers and children in the United states did not observe consistent associations between maternal serum paraxanthine concentrations, the primary metabolite of caffeine, during pregnancy and childhood body mass index at the ages of 4 and 7 years (14). In addition, animal studies have shown a decreased expression of insulin-like growth factor-1 (IGF-1), IGF-1 receptors and insulin receptors in offspring of rats exposed to caffeine during pregnancy, suggesting that fetal exposure to caffeine may disturb early growth and glucose metabolism (15,16). To the best of our knowledge, no previous studies have assessed the associations of maternal caffeine intake during pregnancy with early growth, detailed body fat outcomes or insulin levels in childhood.
Therefore, we examined in a population-based prospective cohort study from early pregnancy onwards among 7,857 mothers and their children, the associations of maternal caffeine intake from coffee and tea during pregnancy with repeatedly measured growth characteristics from birth until the age of 6 years, and detailed body fat measures and insulin and c-peptide levels at the age of 6 years.
Methods
Study design
This study was embedded in the Generation R Study, a population-based prospective cohort study from fetal life until young adulthood performed in Rotterdam, the Netherlands (17,18). Pregnant women were enrolled between 2001 and 2005. Of all eligible children, 61% participated in the study at birth. The study was approved by the local Medical Ethical Committee (MEC 198.782/2001/31). Written informed consent was obtained from all mothers.
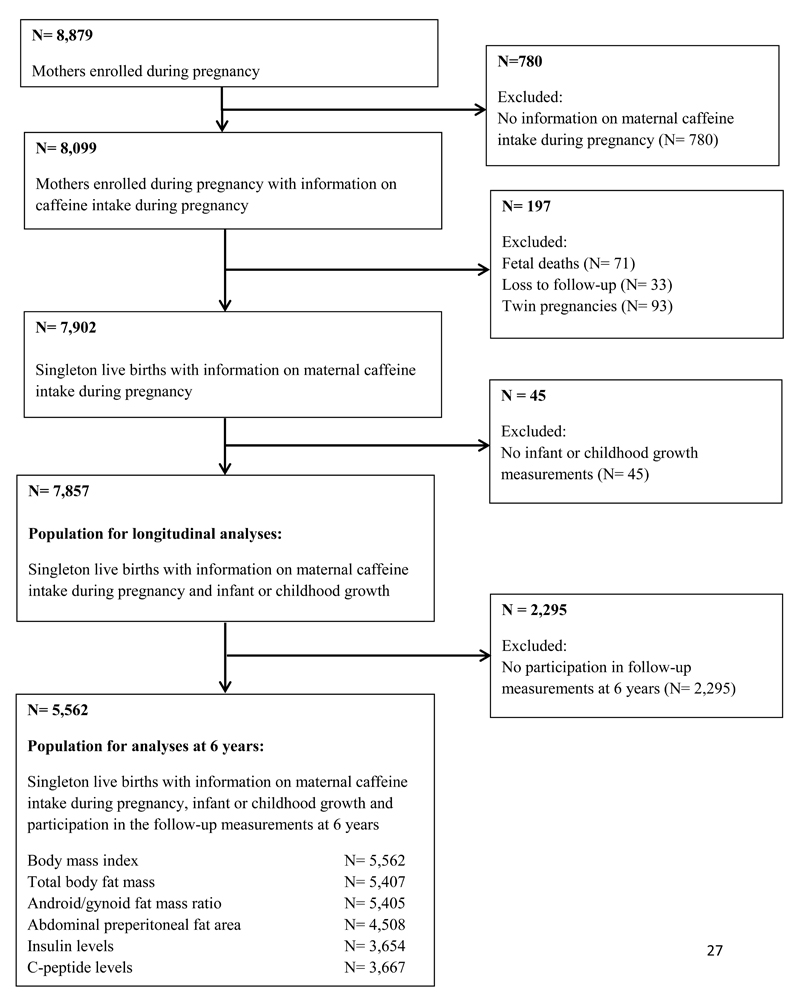
In total, 8,879 mothers were enrolled during pregnancy, of whom 8,099 had information available on maternal caffeine intake during pregnancy. Of their children, 7,902 were singleton and live-born, 7,857 had data available on infant or childhood growth, and 5,562 participated in the follow-up measurements at 6 years and had data available on body mass index, body fat, insulin or c-peptide levels (Flow-chart is given in Figure 1).
Figure 1. Flow-chart of study participants.
Maternal caffeine intake during pregnancy
Information on maternal caffeine intake during pregnancy was obtained by postal questionnaires in the first, second, and third trimester of pregnancy (7). Response rates for these questionnaires were 91%, 80%, and 77%, respectively (7). Mothers who reported to drink any coffee or tea were asked how many cups of coffee or tea they on average consumed per day and what type of coffee or tea they consumed (caffeinated, decaffeinated or a combination of both). According to standard values for caffeine content, a regular coffee serving (125 mL) in the Netherlands contains ~90 mg caffeine, decaffeinated coffee contains ~3 mg, and tea contains ~45 mg (19). To calculate the total caffeine intake in each trimester, the type of coffee or tea was weighted according to its caffeine content (caffeinated coffee = 1, caffeinated and decaffeinated coffee = 0.5, decaffeinated coffee = 0, caffeinated tea = 0.5, caffeinated and decaffeinated tea = 0.25, decaffeinated tea = 0; herbal tea = 0, and green tea = 0.5)(7). Thus, in our analyses, each unit of caffeine intake reflects caffeine exposure based on 1 cup of caffeinated coffee (90 mg caffeine). Total maternal caffeine intake was subsequently categorized (<2, 2-3.9, 4-5.9, ≥6 units per day, equivalent to <180, 180-359, 360-539 and ≥540 mg per day, respectively). The average maternal caffeine intake of the trimesters of pregnancy was used for further analyses. When we used maternal caffeine intake in each trimester separately, results were similar (results not shown).
Infant and childhood growth
Information about length and weight at birth was obtained from medical records. Infant and childhood height and weight were measured using standardized methods at the ages of 6, 12, 24, 36, 48 and 72 months. We calculated body mass index (kg/m2) from the age of 6 months onwards. We created age- and sex-adjusted standard deviation scores (SDS) within our study population using North-European reference growth charts for birth measurements (20) and Dutch reference growth charts for infant and childhood measurements (Growth Analyzer 3.5, Dutch Growth Research Foundation)(21). We defined childhood overweight or obesity at the age of 72 months using the International Obesity Task Force cut offs (boys: body mass index ≥17.55 and ≥19.78, girls: body mass index ≥17.37 and ≥19.65 for overweight and obesity, respectively) (22).
Childhood body fat distribution
At the age of 6 years, we measured total and regional body fat mass using Dual-Energy X-ray absorptiometry (DXA) (iDXA, General Electrics –Lunar, 2008, Madison, WI, USA) (23). Total body fat mass was calculated as a percentage of total body weight measured by DXA. Android/gynoid fat mass ratio was calculated (23). Preperitoneal fat mass was used as a proxy for visceral fat and was measured using abdominal ultrasound examinations with ultrasound LOGIQ E9 (GE Medical System, Wauwatosa, WI, USA) and ATL-Philips Model HDI 5000 (Seattle, WA, USA), as described in detail previously (24). Briefly, a linear (L12-5 MHz) transducer was placed perpendicular to the skin surface on the median upper abdomen (25). We scanned longitudinally from the xiphoid process to the navel along the midline (linea alba). Preperitoneal fat mass was measured as areas of 2 cm length along the midline starting from the reference point in direction of the navel.
Childhood insulin and c-peptide levels
Childhood insulin (pmol/L) and c-peptide levels (nmol/L) were obtained enzymatically from 30 minutes fasting venous blood samples at the age of 6 years using a Cobas 8000 analyzer (Roche, Almere, The Netherlands). Quality control samples demonstrated intra and inter assay coefficients of variation of 1.39% and 2.40%, respectively.
Covariates
We assessed maternal age, pre-pregnancy body mass index, parity, ethnicity, educational level and folic acid supplementation use by questionnaire at enrolment in the study. Smoking and alcohol consumption during pregnancy were repeatedly assessed by questionnaire. We obtained information on gestational hypertensive disorders (gestational hypertension and pre-eclampsia) and gestational diabetes, date of birth and the child’s sex from midwife and hospital registries. We obtained information on breastfeeding and the timing of introduction to solid foods by questionnaire during infancy. Average television-watching time was assessed by questionnaire at the age of 6 years.
Statistical analysis
First, we used unbalanced repeated measurement regression models to examine the associations of maternal caffeine intake during pregnancy with longitudinally measured growth characteristics. These models take the correlation between repeated measurements of the same subject into account and allow for incomplete outcome data. The models are described in more detail in Supporting Information Methods S1.These models were adjusted for child’s sex, and maternal and childhood sociodemographic and lifestyle related characteristics.
Second, we used multiple linear regression models to examine the associations of maternal caffeine intake during pregnancy with childhood body fat distribution and insulin and c-peptide levels. These models were first adjusted for child’s sex, age at follow-up measurement and height at follow-up measurement (for fat mass outcomes only) and subsequently additionally adjusted for maternal and childhood sociodemographic and lifestyle related characteristics. We included covariates in the models based on their associations with the outcomes of interest in previous studies, a significant association with the determinants and outcomes, or a change in effect estimates of >10%. To examine whether a dose-response relationship is present, we performed tests for trends by entering the categorized variable as a continuous term to the models. Finally, we used logistic regression models to examine the associations of maternal caffeine intake during pregnancy with childhood overweight at the age of 6 years using similar adjustments.
In order to obtain normal distributions, we log transformed fat mass outcomes and square root transformed insulin and c-peptide levels. We constructed standard deviation scores (SDS) for all outcomes. Since no significant interaction between maternal caffeine intake during pregnancy and child’s sex was present (all p-values >0.05), we performed no sex-stratified analyses. We used Multiple Imputation for missing values of covariates, by generating 5 independent datasets using the Markov Chain Monte Carlo (MCMC) method (26). We included all covariates in the imputation model. In addition, childhood body mass index and insulin levels at the age of 6 years, maternal pre-pregnancy weight, maternal height, income, maternal caffeine intake and paternal body mass index were used as predictors only, and were not imputed themselves. Percentages missing values in the population for analysis were all lower than 22%, except for timing of introduction of solid foods (37.6%). Pooled effect estimates were presented.The repeated measurement analysis was performed using the Statistical Analysis System version 9.3 (SAS, Institute Inc. Cary NC). All other analyses were performed using the Statistical Package of Social Sciences version 22.0 for Windows (IBM Corp., Armonk, NY, USA).
Results
Study population
Table 1 shows that, as compared to mothers who consumed <2 units of caffeine per day during their pregnancy, those who consumed ≥6 units per day were more likely to be higher educated, nulliparous and from European descent. Their children had a lower birth weight and a higher body mass index at the age of 6 years (p-values<0.05). Supporting Information Table S1 shows infant and childhood growth characteristics. Supporting Information Table S2 shows limited to moderate correlations between the outcome measures at 6 years. Non-response analyses at baseline (Supporting Information Table S3) and at follow-up measurement (Supporting Information Table S4) showed that both mothers excluded because of missing data on caffeine intake during pregnancy and mothers lost to follow-up were lower educated and less often of European descent, compared to those included in the analysis. Their children had a lower birth weight. However, no large differences were observed between the caffeine intake during pregnancy of mothers of children not included in the analyses at 6 years and mothers of children included in the analyses (median (95% range) caffeine intake: 1.3 units (0, 5.0) vs. 1.5 units (0, 5.0)).
Table 1. Characteristics of the mothers and their children (N=7,857)1.
| Maternal caffeine intake during pregnancy2 | ||||||
|---|---|---|---|---|---|---|
| Total group N=7,857 |
<2 units N=4,804 |
2-3.9 units N=2,447 |
4-5.9 units N=496 |
>6 units N=110 |
P-value3 | |
| Maternal characteristics | ||||||
| Age, median (95% range), years | 30.4 (19.3, 39.2) | 29.3 (18.7, 38.8) | 31.5 (20.5, 39.9) | 32.6 (21.2, 40.3) | 32.8 (22.2, 40.8) | <0.001 |
| Height, mean (SD), cm | 167.4 (7.4) | 166.6 (7.4) | 168.4 (7.2) | 169.4 (7.3) | 167.9 (7.1) | <0.001 |
| Pre-pregnancy weight, median (95% range), kg | 64.0 (48.0, 99.0) | 63.0 (48.0, 100.0) | 65.0 (49.0, 95.0) | 65.0 (49.0, 100.1) | 67.5 (48.1, 100.3) | 0.076 |
| Pre-pregnancy BMI, median (95% range), kg/m2 | 22.6 (17.9, 35.0) | 22.6 (17.8, 35.6) | 22.6 (18.1, 34.1) | 22.6 (18.0, 35,5) | 22.8 (18.4, 33.0) | 0.108 |
| Education, No. (%) | ||||||
| Primary | 421 (5.9) | 295 (6.8) | 106 (4.8) | 18 (4.0) | 2 (1.9) | <0.001 |
| Secondary | 3,070 (43.3) | 2,021 (46.8) | 814 (36.6) | 176 (39.5) | 59 (56.7) | |
| Higher | 3,600 (50.8) | 2,003 (46.4) | 1,302 (58.6) | 252 (56.5) | 43 (41.3) | |
| Parity, No. nulliparous (%) | 4,405 (56.4) | 2,838 (59.5) | 1,278 (52.4) | 243 (49.2) | 46 (42.2) | <0.001 |
| Ethnicity, No. European (%) | 4,485 (58.5) | 2,387 (51.1) | 1,636 (67.9) | 378 (77.6) | 84 (77.1) | <0.001 |
| Folic acid supplementation use, No. Yes (%) | 4,375 (71.3) | 2,558 (69.1) | 1,466 (75.1) | 302 (75.7) | 49 (61.3) | <0.001 |
| Smoking during pregnancy, No. Yes (%) | 1,336 (18.6) | 645 (14.7) | 493 (22.0) | 144 (31.7) | 54 (51.4) | <0.001 |
| Alcohol consumption during pregnancy, No. Yes (%) | 2,672 (37.6) | 1,339 (30.7) | 1,062 (48.0) | 215 (48.8) | 56 (54.4) | <0.001 |
| Gestational diabetes, No. Yes (%) | 80 (1.1) | 48 (1.0) | 25 (1.1) | 7 (1.5) | 0 (0.0) | N/A4 |
| Pre-eclampsia, No. Yes (%) | 153 (2.1) | 99 (2.2) | 37 (1.6) | 16 (3.4) | 1(1.0) | 0.072 |
| Gestational hypertension, No Yes (%) | 293 (3.9) | 187 (4.1) | 87 (3.7) | 15 (3.2) | 4 (3.8) | 0.775 |
| Child characteristics | ||||||
| Males, No. (%) | 3,957 (50.4) | 2,452 (51.1) | 1,176 (48.1) | 267 (53.8) | 62 (56.4) | 0.018 |
| Gestational age at birth, median (95% range), weeks | 40.1 (35.7, 42.3) | 40.1 (35.6, 42.3) | 40.1 (36.0, 42.3) | 40.3 (33.3, 42.4) | 40.2 (33.3, 42.5) | 0.001 |
| Birth weight, median (95% range), g | 3435 (2242, 4490) | 3420 (2250, 4450) | 3460 (2260, 4520) | 3490 (2144, 4567) | 3380 (1980, 4300) | <0.001 |
| Ever breastfeeding, No. Yes (%) | 5,282 (91.9) | 3,131 (92.1) | 1,733 (92.0) | 353 (90.7) | 65 (87.8) | 0.467 |
| Introduction of solid foods, No. before 6 months (%) | 3,859 (89.5) | 2,208 (88.9) | 1,321 (90.0) | 282 (91.6) | 48 (92.3) | 0.349 |
| Age at 6 year follow-up measurement, median (95% range), years | 6.0 (5.6, 7.9) | 6.0 (5.6, 8.0) | 6.0 (5.6, 7.8) | 6.0 (5.5, 7.4) | 6.1 (5.6, 7.5) | 0.042 |
| Television watching, No. More than 2 hours/day (%) | 934 (19.2) | 588 (21.0) | 284 (17.3) | 47 (13.4) | 15 (23.4) | 0.001 |
| Body mass index at 6 years, median (95% range), kg/m2 | 15.9 (13.6, 21.3) | 15.9 (13.6, 21.3) | 15.7 (13.7, 20.9) | 16.0 (13.8, 21.4) | 16.2 (13.9, 21.0) | 0.001 |
| Overweight at 6 years, No. Yes (%) | 991 (17.8) | 633 (19.2) | 280 (15.4) | 63 (17.1) | 15 (18.8) | 0.008 |
| Total body fat mass, median (95% range), % | 24.0 (16.3, 38.8) | 24.1 (16.2, 39.2) | 23.9 (16.6, 38.2) | 24.0 (16.3, 38.2) | 23.2 (15.8, 41.6) | 0.198 |
| Android/gynoid fat mass ratio, median (95% range) | 0.24 (0.16, 0.42) | 0.24 (0.16, 0.42) | 0.24 (0.16, 0.42) | 0.24 (0.16, 0.43) | 0.25 (0.17, 0.43) | 0.197 |
| Abdominal preperitoneal fat area, median (95% range), cm2 | 0.39 (0.16, 1.21) | 0.40 (0.16, 1.27) | 0.39 (0.16, 1.15) | 0.39 (0.15, 1.03) | 0.33 (0.14, 1.19) | 0.002 |
| Insulin, median (95% range), (pmol/L) | 115 (17, 402) | 115 (16, 395) | 114 (18, 408) | 132 (16, 467) | 91 (14, 369) | 0.015 |
| C-peptide median (95% range), (nmol/L) | 0.96 (0.30, 2.14) | 0.97 (0.30, 2.15) | 0.95 (0.32, 2.09) | 1.06 (0.26, 2,48) | 0.88 (0.36, 2,16) | 0.039 |
Values represent means (SD), median (95% range) or number of subjects (valid %).
1 unit of caffeine intake represents the equivalent of 1 cup of coffee (90 mg caffeine).
Differences in subject characteristics between the groups were tested using One-Way ANOVA for continuous variables and Chi-square tests for proportions.
Not available as a result of small numbers.
Infant and childhood growth patterns
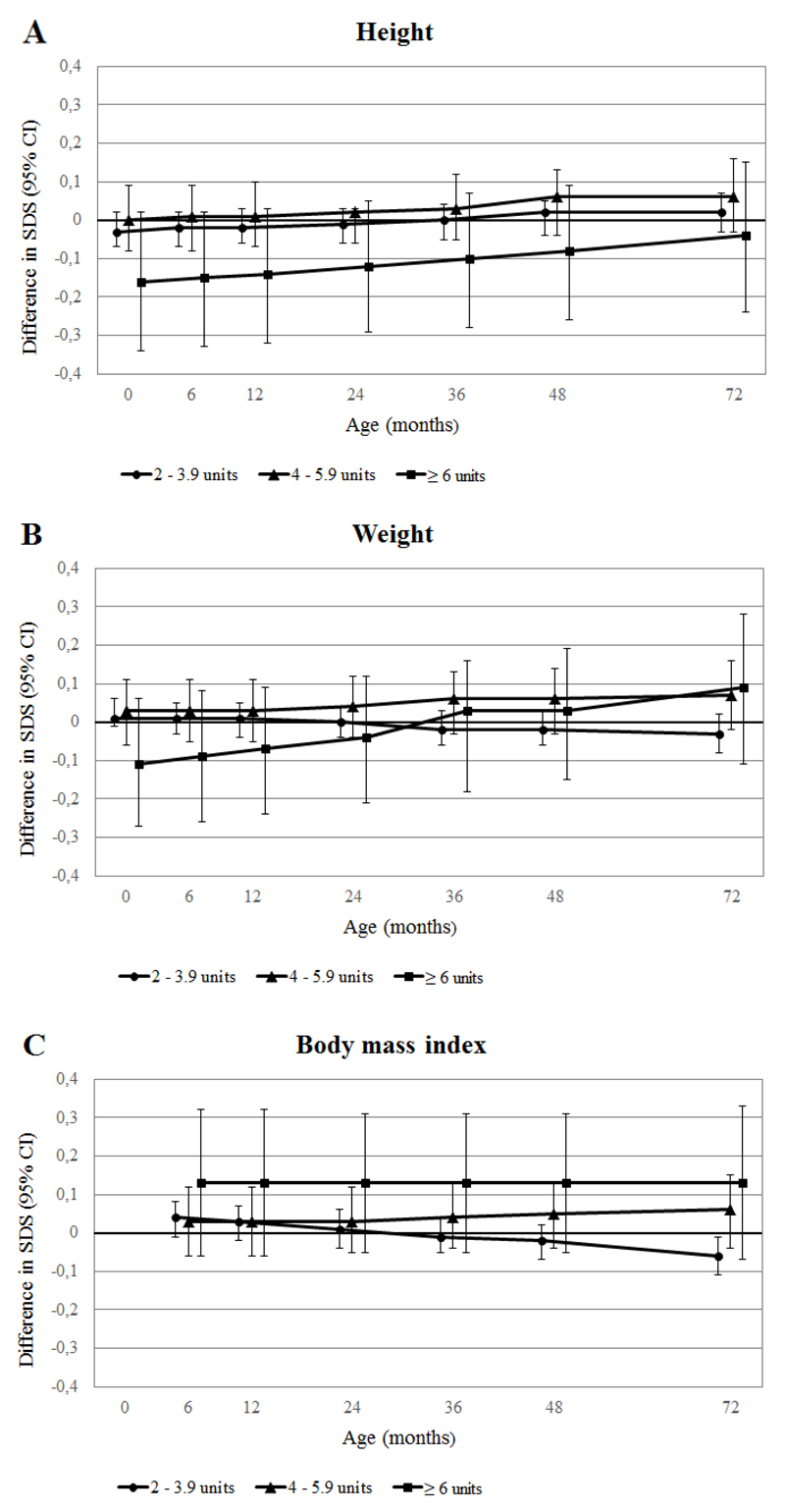
Figure 2 (A-C) shows the associations of maternal caffeine intake during pregnancy with repeatedly measured growth characteristics from birth to 72 months, obtained from repeated measurement regression models. As compared to children whose mothers consumed <2 units of caffeine per day during pregnancy, children whose mothers consumed ≥6 units of caffeine per day were shorter, but these differences decreased over time. At the age of 6 years, children whose mothers consumed ≥6 units of caffeine per day still tended to be shorter. These children also had lower birth weights and had higher weight gain from birth to 72 months. Body mass index tended to be higher from 6 months to 72 months in children whose mothers consumed ≥6 units of caffeine per day during pregnancy, as compared to children whose mothers consumed <2 units of caffeine per day.
Figure 2. Associations of maternal caffeine intake during pregnancy with longitudinally measured growth characteristics (N=7,857).
Results are based on repeated linear regression models and reflect the differences in SDS of (A) height (based on 51,691 measurements), (B) weight (based on 58,124 measurements) and (C) body mass index (based on 36,953 measurements) growth in children whose mothers consumed 2-3.9, 4-5.9 and ≥6 units of caffeine per day during pregnancy, respectively, as compared to those whose mothers consumed <2 units of caffeine per day. 1 unit of caffeine intake represents the equivalent of 1 cup of coffee (90 mg caffeine). The reference value is an SDS of 0. The models were adjusted for child’s sex, maternal age, pre-pregnancy body mass index, parity, ethnicity, educational level, folic acid supplementation use, smoking and alcohol consumption during pregnancy, pregnancy complications, breastfeeding and timing of introduction of solid foods. All p-values for interaction <0.05.
Childhood body fat distribution
Compared to children whose mothers consumed <2 units of caffeine per day during their pregnancy, both those whose mothers consumed 4-5.9 units and ≥6 units of caffeine per day tended to have a higher childhood body mass index (differences: 0.09 Standard deviation score (SDS) (95% Confidence Interval (CI): -0.01, 0.19) and 0.16 SDS (95% CI: -0.03, 0.36), respectively) and a higher childhood total body fat mass (differences: 0.10 SDS (95% CI: 0.01, 0.20) and 0.18 SDS (95% CI: -0.01, 0.37), respectively) (Table 2). Only children whose mothers consumed ≥6 units of caffeine per day during their pregnancy had a higher childhood android/gynoid fat mass ratio (difference: 0.27 SDS (95% CI: 0.05, 0.49)). Similar tendencies were present when we combined the upper two maternal caffeine intake categories into one category (results not shown). Supporting Information Table S5 shows similar results from the basic models. Supporting Information Table S6 shows that as compared to children whose mothers consumed <2 units of caffeine per day during pregnancy, those whose mothers consumed ≥6 units of caffeine per day tended to have higher risks of childhood overweight (Odds Ratio (OR): 1.25 (95% CI: 0.68, 2.30) in the fully adjusted model.
Table 2. Maternal caffeine intake during pregnancy and childhood body fat distribution at 6 years (fully adjusted models) (N=5,562).
| Body mass index (SDS) | Total body fat mass (SDS) | Android/gynoid fat mass ratio (SDS) | Abdominal preperitoneal fat area (SDS) | |
|---|---|---|---|---|
| N=5,562 | N=5,407 | N=5,405 | N=4,508 | |
| Maternal caffeine intake categories | ||||
| < 2 units | Reference N=3,295 |
Reference N= 3,199 |
Reference N=3,197 |
Reference N=2,658 |
| 2 – 3.9 units | -0.03 (-0.08, 0.03) N= 1,819 |
0.02 (-0.03, 0.07) N=1,771 |
0.04 (-0.02, 0.09) N=1,771 |
-0.05 (-0.11, 0.01) N= 1,475 |
| 4 – 5.9 units | 0.09 (-0.01, 0.19) N=368 |
0.10 (0.01, 0.20)* N=357 |
0.02 (-0.09, 0.13) N=357 |
-0.04 (-0.15, 0.07) N=306 |
| ≥ 6 units | 0.16 (-0.03, 0.36) N=80 |
0.18 (-0.01, 0.37) N=80 |
0.27 (0.05, 0.49)* N=80 |
-0.15 (-0.37, 0.07) N=69 |
| P-value for trend | 0.220 | 0.015 | 0.068 | 0.075 |
Values are regression coefficients (95% confidence interval) that reflect the difference in childhood outcomes in children whose mothers consumed 2-3.9, 4-5.9 and ≥6 units of caffeine per day during pregnancy, respectively, as compared to those whose mothers consumed <2 units of caffeine per day. 1 unit of caffeine intake represents the equivalent of 1 cup of coffee (90 mg caffeine). The models were adjusted for child’s sex, age at follow-up measurement, height at follow-up measurement, maternal age, pre-pregnancy body mass index, parity, ethnicity, educational level, folic acid supplementation use, smoking and alcohol consumption during pregnancy, gestational diabetes, gestational hypertensive disorders, birth weight, gestational age at birth, breastfeeding, introduction of solid foods and television-watching time. P-values for trend were obtained from models in which the categorized caffeine intake variable was entered as continuous variable.
P-value <0.05.
Childhood insulin and c-peptide levels
Table 3 shows no consistent associations of maternal caffeine intake during pregnancy with childhood insulin and c-peptide levels in the fully adjusted models. Similar results were present in the basic models (Supporting Information Table S7).
Table 3. Maternal caffeine intake during pregnancy and childhood insulin and c-peptide levels at 6 years (fully adjusted models) (N=3,667).
| Insulin (SDS) | C-peptide (SDS) | |
|---|---|---|
| N=3,654 | N=3,667 | |
| Maternal caffeine intake categories | ||
| < 2 units | Reference N= 2,116 |
Reference N=2,128 |
| 2 -3.9 units | -0.03 (-0.11, 0.04) N= 1,239 |
-0.05 (-0.12, 0.03) N=1,237 |
| 4 – 5.9 units | 0.14 (0.01, 0.28)* N= 241 |
0.10 (-0.03, 0.24) N=243 |
| ≥ 6 units | -0.18 (-0.45, 0.08) N= 58 |
-0.13 (-0.39, 0.14) N=59 |
| P-value for trend | 0.900 | 0.869 |
Values are regression coefficients (95% confidence interval) that reflect the difference in childhood outcomes in children whose mothers consumed 2-3.9, 4-5.9 and ≥6 units of caffeine per day during pregnancy, respectively, as compared to those whose mothers consumed <2 units of caffeine per day. 1 unit of caffeine intake represents the equivalent of 1 cup of coffee (90 mg caffeine). The models were adjusted for child’s sex, age at follow-up measurement, maternal age, pre-pregnancy body mass index, parity, ethnicity, educational level, folic acid supplementation use, smoking and alcohol consumption during pregnancy, gestational diabetes, gestational hypertensive disorders, birth weight, gestational age at birth, breastfeeding, introduction of solid foods and television-watching time. P-values for trend were obtained from models in which the categorized caffeine intake variable was entered as continuous variable.
P-value <0.05.
Discussion
We observed that, as compared to children whose mothers consumed no or less than 2 units of caffeine per day during their pregnancy, children whose mothers consumed 6 units of caffeine or more per day tended to have a lower weight at birth, higher weight gain from birth to 6 years and a higher body mass index from 6 months to 6 years. Also, at the age of 6 years, children of mothers with higher levels of caffeine intake during pregnancy tended to have a higher childhood total body fat mass and android/gynoid fat mass ratio. We did not observe differences for childhood insulin or c-peptide levels.
Strengths and limitations
We used a large population-based cohort followed from early pregnancy onwards. In total, 30% of the eligible participants with information on maternal caffeine intake during pregnancy were not participating in follow-up measurements at 6 years. This loss to follow-up could have reduced the statistical power of our study and could have led to biased effect estimates if associations of interest differ between children included and not included in the analysis. This seems unlikely, since only minor differences were observed between the caffeine intake during pregnancy of mothers of children not included in the analysis and the caffeine intake of mothers of children included in the analysis. Since maternal caffeine intake during pregnancy was self-reported, misclassification by underreporting may be present. In addition, in accordance with the Netherlands Nutrition Centre (27), we assumed that coffee was consumed in cups of 125 mL. However, this might have differed between participants, which may have led to some misclassification of the categories of maternal caffeine intake. We also assessed only caffeine intake from coffee and tea and not intake from other sources, such as soft drinks, chocolate and medications. However, at the time of data collection (2002-2006), coffee and tea accounted for 70 and 26% , respectively, of all caffeine ingested (1). We categorized maternal caffeine intake during pregnancy in units of caffeine instead of calculating the exact milligrams of caffeine consumed per day. The highest category of maternal caffeine intake in our study (≥6 units) should be considered equivalent to a caffeine intake of ≥540 mg per day. However, since caffeine contents per unit of coffee might differ between countries, our results should be interpreted carefully with regard to other populations. We were able to adjust our analyses for many possible confounders. However, as in any observational study, residual confounding might still be an issue. For example, in our study we were unable to adjust the analyses for detailed maternal and childhood dietary habits.
Interpretation of main findings
Maternal caffeine intake during pregnancy may affect fetal growth and development. Among the same population as the present study and in line with other large observational studies (4–6), we previously reported that high levels of maternal caffeine intake during pregnancy are associated with impaired fetal growth and higher risks of low birth weight (7).
Not much is known about the long-term offspring consequences of maternal caffeine intake during pregnancy. A recent study in the United States among 615 mothers and their children suggested that any caffeine intake during pregnancy was associated with an 87% higher overall risk of childhood obesity before the age of 15 years. Also, a dose-response relation for maternal caffeine intake was observed (13). In contrast, another recent study among 1986 mothers and their children in the United States did not observe consistent associations of maternal serum paraxanthine concentrations, caffeine’s primary metabolite, during pregnancy with childhood body mass index (14). We observed that children of mothers with the highest caffeine intake during pregnancy were shorter and had lower weights at birth, as compared to children of mothers with low caffeine intake, but gained more weight from birth to 6 years. Also, they tended to have higher body mass indexes from 6 months to 6 years. We observed a tendency to a higher risk of overweight at the age of 6 years in children of mothers with the highest caffeine intake during pregnancy, although not statistically significant. This may be due to smaller numbers, since only 80 mothers in our study consumed 6 units of caffeine per day or more. Thus, these findings suggest that maternal caffeine intake during pregnancy might not only affect fetal development, but may have persistent consequences for childhood growth.
Although body mass index is a widely accepted measure of adiposity, previous studies have shown that more specific body fat measures, such as total fat mass, waist circumference and waist to hip ratio, are predictors of cardiovascular risk factors and disease in children and adults, independent of body mass index (28–30). Thus, detailed body composition measures provide useful additional information in assessing adiposity and its consequences. To the best of our knowledge, no previous studies have been performed focused on the associations of maternal caffeine intake during pregnancy with detailed measures of childhood body fat distribution. We observed that high maternal caffeine intake during pregnancy tended to be associated with a higher childhood total body fat mass and an adverse childhood body fat distribution, as reflected by a higher android/gynoid fat mass ratio. We observed no effect of maternal caffeine intake during pregnancy on preperitoneal fat mass. This discrepancy could be attributed to a larger measurement error for preperitoneal fat mass measurements in childhood (31,32). Thus, our results suggest that maternal caffeine intake during pregnancy may affect childhood total fat and body fat distribution, next to body mass index.
Studies in adults showed that coffee consumption is consistently associated with lower risks of insulin resistance and type 2 diabetes (33–35). However, associations with both caffeinated and decaffeinated coffee were observed, suggesting that next to caffeine, other coffee components may also play a role in the underlying mechanisms. In contrast to these findings in non-pregnant adults, animal studies suggest that maternal caffeine intake during pregnancy may increase insulin resistance and disturb glucose metabolism in the offspring (15,16). We did not observe consistent associations of maternal caffeine intake during pregnancy with childhood insulin and c-peptide levels. Our results should be interpreted carefully, since the fasting period before blood draw for the childhood insulin and c-peptide measurements was limited. This may have led to some non-differential misclassification and an underestimation of the observed effect estimates. This may especially affect childhood insulin levels, which are less stable and have a shorter half-life as compared to c-peptide levels. Further studies are needed to assess the detailed associations of maternal caffeine intake during pregnancy with offspring glucose and insulin metabolism.
The mechanisms by which maternal caffeine intake during pregnancy might influence childhood body fat distribution are not clear. It has been suggested that caffeine induces an increase in circulating maternal and fetal glucocorticoid concentrations (36,37). Studies in rats suggest that fetal overexposure to glucocorticoids leads to an altered development of the hypothalamic-pituitary-adrenal axis (HPA-axis), impaired fetal growth, altered structure of the endocrine pancreas, insulin target-tissues and adipose depots and increased HPA-axis activity (37–39). Whether this mechanism partly underlies the observed associations needs to be further studied.
Although the observed effect estimates are small and without direct individual clinical consequence, our results suggest that maternal caffeine intake during pregnancy is associated with infant and childhood growth and body fat distribution. As caffeine is frequently consumed during pregnancy and the prevalence of obesity is still rising (40), our results underline the need to study the long-term health consequences of maternal caffeine intake during pregnancy.
Conclusion
Our results suggest that high levels of maternal caffeine intake during pregnancy are associated with adverse offspring growth patterns and childhood body fat distribution, but not with childhood insulin and c-peptide levels. Further studies are needed to assess whether maternal caffeine intake during pregnancy affects long-term offspring health outcomes, and the causality and underlying mechanisms.
Supplementary Material
What is already known about this subject?
High levels of maternal caffeine intake during pregnancy are associated with higher risks of low birth weight.
Children born with a low birth weight are at higher risk of an adverse body fat distribution and insulin resistance in later life.
What does our study add?
Our results suggest that high levels of maternal caffeine intake during pregnancy are associated with adverse infant and childhood growth patterns and a higher body mass index, total body fat mass and android/gynoid fat mass ratio at school-age.
Acknowledgments
The Generation R Study is conducted by the Erasmus Medical Center in close collaboration with the School of Law and Faculty of Social Sciences of the Erasmus University Rotterdam, the Municipal Health Service Rotterdam area, Rotterdam, the Rotterdam Homecare Foundation, Rotterdam and the Stichting Trombosedienst and Artsenlaboratorium Rijnmond (STAR), Rotterdam. We gratefully acknowledge the contribution of participating mothers, general practitioners, hospitals, midwives and pharmacies in Rotterdam.
Funding: The Generation R Study is financially supported by the Erasmus Medical Center, Rotterdam, the Erasmus University Rotterdam and the Netherlands Organization for Health Research and Development. Vincent Jaddoe received an additional grant from the Netherlands Organization for Health Research and Development (NWO, ZonMw-VIDI 016.136.361) and an European Research Council Consolidator Grant (ERC-2014-CoG-648916). Oscar Franco works in ErasmusAGE, a center for aging research across the life course funded by Nestlé Nutrition (Nestec Ltd.); Metagenics Inc.; and AXA. Nestlé Nutrition (Nestec Ltd.); Metagenics Inc.; and AXA had no role in design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review or approval of the manuscript. The research leading to these results has received funding from the European Union’s Seventh Framework Programme (FP7/2007-2013), project EarlyNutrition under grant no289346.
Footnotes
Disclosure: The authors declare no conflict of interest.
References
- 1.Clausson B, Granath F, Ekbom A, et al. Effect of caffeine exposure during pregnancy on birth weight and gestational age. Am J Epidemiol. 2002;155:429–36. doi: 10.1093/aje/155.5.429. [DOI] [PubMed] [Google Scholar]
- 2.Goldstein A, Warren R. Passage of caffeine into human gonadal and fetal tissue. Biochem Pharmacol. 1962;11:166–8. doi: 10.1016/0006-2952(62)90106-5. [DOI] [PubMed] [Google Scholar]
- 3.Brent RL, Christian MS, Diener RM. Evaluation of the reproductive and developmental risks of caffeine. Birth Defects Res B Dev Reprod Toxicol. 2011;92:152–87. doi: 10.1002/bdrb.20288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen LW, Wu Y, Neelakantan N, Chong M, Pan A, van Dam RM. Maternal caffeine intake during pregnancy is associated with risk of low birth weight: a systematic review and dose-response meta-analysis. BMC Med. 2014;12:174. doi: 10.1186/s12916-014-0174-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sengpiel V, Elind E, Bacelis J, et al. Maternal caffeine intake during pregnancy is associated with birth weight but not with gestational length: results from a large prospective observational cohort study. BMC Med. 2013;11:42. doi: 10.1186/1741-7015-11-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.CARE Study Group. Maternal caffeine intake during pregnancy and risk of fetal growth restriction: a large prospective observational study. BMJ. 2008;337:a2332. doi: 10.1136/bmj.a2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bakker R, Steegers EA, Obradov A, Raat H, Hofman A, Jaddoe VW. Maternal caffeine intake from coffee and tea, fetal growth, and the risks of adverse birth outcomes: the Generation R Study. Am J Clin Nutr. 2010;91:1691–8. doi: 10.3945/ajcn.2009.28792. [DOI] [PubMed] [Google Scholar]
- 8.Ong KK, Ahmed ML, Emmett PM, Preece MA, Dunger DB. Association between postnatal catch-up growth and obesity in childhood: prospective cohort study. BMJ. 2000;320:967–71. doi: 10.1136/bmj.320.7240.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ibanez L, Ong K, Dunger DB, de Zegher F. Early development of adiposity and insulin resistance after catch-up weight gain in small-for-gestational-age children. J Clin Endocrinol Metab. 2006;91:2153–8. doi: 10.1210/jc.2005-2778. [DOI] [PubMed] [Google Scholar]
- 10.Harder T, Rodekamp E, Schellong K, Dudenhausen JW, Plagemann A. Birth weight and subsequent risk of type 2 diabetes: a meta-analysis. Am J Epidemiol. 2007;165:849–57. doi: 10.1093/aje/kwk071. [DOI] [PubMed] [Google Scholar]
- 11.Whincup PH, Kaye SJ, Owen CG, et al. Birth weight and risk of type 2 diabetes: a systematic review. JAMA. 2008;300:2886–97. doi: 10.1001/jama.2008.886. [DOI] [PubMed] [Google Scholar]
- 12.Barker DJ. Adult consequences of fetal growth restriction. Clin Obstet Gynecol. 2006;49:270–83. doi: 10.1097/00003081-200606000-00009. [DOI] [PubMed] [Google Scholar]
- 13.Li DK, Ferber JR, Odouli R. Maternal caffeine intake during pregnancy and risk of obesity in offspring: a prospective cohort study. Int J Obes (Lond) 2014;39:658–64. doi: 10.1038/ijo.2014.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klebanoff MA, Keim SA. Maternal serum paraxanthine during pregnancy and offspring body mass index at ages 4 and 7 years. Epidemiology. 2015;26:185–91. doi: 10.1097/EDE.0000000000000220. [DOI] [PubMed] [Google Scholar]
- 15.Tan Y, Liu J, Deng Y, et al. Caffeine-induced fetal rat over-exposure to maternal glucocorticoid and histone methylation of liver IGF-1 might cause skeletal growth retardation. Toxicol Lett. 2012;214:279–87. doi: 10.1016/j.toxlet.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 16.Liu Y, Xu D, Feng J, et al. Fetal rat metabonome alteration by prenatal caffeine ingestion probably due to the increased circulatory glucocorticoid level and altered peripheral glucose and lipid metabolic pathways. Toxicol Appl Pharmacol. 2012;262:205–16. doi: 10.1016/j.taap.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 17.Jaddoe VW, van Duijn CM, Franco OH, et al. The Generation R Study: design and cohort update 2012. Eur J Epidemiol. 2012;27:739–56. doi: 10.1007/s10654-012-9735-1. [DOI] [PubMed] [Google Scholar]
- 18.Kruithof CJ, Kooijman MN, van Duijn CM, et al. The Generation R Study: Biobank update 2015. Eur J Epidemiol. 2014;29:911–27. doi: 10.1007/s10654-014-9980-6. [DOI] [PubMed] [Google Scholar]
- 19.NEVO-tabel 2006. [Dutch Food Composition Table 2006] The Hague, Netherlands: Voedingscentrum; [Netherlands Nutrition Center]; 2006. (in Dutch) [Google Scholar]
- 20.Niklasson A, Ericson A, Fryer JG, Karlberg J, Lawrence C, Karlberg P. An update of the Swedish reference standards for weight, length and head circumference at birth for given gestational age (1977-1981) Acta Paediatr Scand. 1991;80:756–62. doi: 10.1111/j.1651-2227.1991.tb11945.x. [DOI] [PubMed] [Google Scholar]
- 21.Fredriks AM, van Buuren S, Burgmeijer RJ, et al. Continuing positive secular growth change in The Netherlands 1955-1997. Pediatr Res. 2000;47:316–23. doi: 10.1203/00006450-200003000-00006. [DOI] [PubMed] [Google Scholar]
- 22.Cole TJ, Bellizzi MC, Flegal KM, Dietz WH. Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ. 2000;320:1240–3. doi: 10.1136/bmj.320.7244.1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Helba M, Binkovitz LA. Pediatric body composition analysis with dual-energy X-ray absorptiometry. Pediatr Radiol. 2009;39:647–56. doi: 10.1007/s00247-009-1247-0. [DOI] [PubMed] [Google Scholar]
- 24.Holzhauer S, Zwijsen RM, Jaddoe VW, et al. Sonographic assessment of abdominal fat distribution in infancy. Eur J Epidemiol. 2009;24:521–9. doi: 10.1007/s10654-009-9368-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Suzuki R, Watanabe S, Hirai Y, et al. Abdominal wall fat index, estimated by ultrasonography, for assessment of the ratio of visceral fat to subcutaneous fat in the abdomen. Am J Med. 1993;95:309–14. doi: 10.1016/0002-9343(93)90284-v. [DOI] [PubMed] [Google Scholar]
- 26.Sterne JA, White IR, Carlin JB, et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ. 2009;338:b2393. doi: 10.1136/bmj.b2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cafeïne. [Caffeine] Voedingscentrum. [The Netherlands Nutrition Centre]: (in Dutch). Available from: http://www.voedingscentrum.nl/encyclopedie/cafeine.aspx. [Google Scholar]
- 28.Srinivasan SR, Wang R, Chen W, Wei CY, Xu J, Berenson GS. Utility of waist-to-height ratio in detecting central obesity and related adverse cardiovascular risk profile among normal weight younger adults (from the Bogalusa Heart Study) Am J Cardiol. 2009;104:721–4. doi: 10.1016/j.amjcard.2009.04.037. [DOI] [PubMed] [Google Scholar]
- 29.Pischon T, Boeing H, Hoffmann K, et al. General and abdominal adiposity and risk of death in Europe. N Engl J Med. 2008;359:2105–20. doi: 10.1056/NEJMoa0801891. [DOI] [PubMed] [Google Scholar]
- 30.Gishti O, Gaillard R, Durmus B, et al. BMI, total and abdominal fat distribution, and cardiovascular risk factors in school-age children. Pediatr Res. 2015;77:710–8. doi: 10.1038/pr.2015.29. [DOI] [PubMed] [Google Scholar]
- 31.Mook-Kanamori DO, Holzhauer S, Hollestein LM, et al. Abdominal fat in children measured by ultrasound and computed tomography. Ultrasound Med Biol. 2009;35:1938–46. doi: 10.1016/j.ultrasmedbio.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 32.Staiano AE, Katzmarzyk PT. Ethnic and sex differences in body fat and visceral and subcutaneous adiposity in children and adolescents. Int J Obes (Lond) 2012;36:1261–9. doi: 10.1038/ijo.2012.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bhupathiraju SN, Pan A, Malik VS, et al. Caffeinated and caffeine-free beverages and risk of type 2 diabetes. Am J Clin Nutr. 2013;97:155–66. doi: 10.3945/ajcn.112.048603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ding M, Bhupathiraju SN, Chen M, van Dam RM, Hu FB. Caffeinated and decaffeinated coffee consumption and risk of type 2 diabetes: a systematic review and a dose-response meta-analysis. Diabetes Care. 2014;37:569–86. doi: 10.2337/dc13-1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van Dam RM, Hu FB. Coffee consumption and risk of type 2 diabetes: a systematic review. JAMA. 2005;294:97–104. doi: 10.1001/jama.294.1.97. [DOI] [PubMed] [Google Scholar]
- 36.Kirkinen P, Jouppila P, Koivula A, Vuori J, Puukka M. The effect of caffeine on placental and fetal blood flow in human pregnancy. Am J Obstet Gynecol. 1983;147:939–42. doi: 10.1016/0002-9378(83)90250-8. [DOI] [PubMed] [Google Scholar]
- 37.Reynolds RM. Corticosteroid-mediated programming and the pathogenesis of obesity and diabetes. J Steroid Biochem Mol Biol. 2010;122:3–9. doi: 10.1016/j.jsbmb.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 38.Xu D, Wu Y, Liu F, et al. A hypothalamic-pituitary-adrenal axis-associated neuroendocrine metabolic programmed alteration in offspring rats of IUGR induced by prenatal caffeine ingestion. Toxicol Appl Pharmacol. 2012;264:395–403. doi: 10.1016/j.taap.2012.08.016. [DOI] [PubMed] [Google Scholar]
- 39.Xu D, Zhang B, Liang G, et al. Caffeine-induced activated glucocorticoid metabolism in the hippocampus causes hypothalamic-pituitary-adrenal axis inhibition in fetal rats. PLoS One. 2012;7:e44497. doi: 10.1371/journal.pone.0044497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Han JC, Lawlor DA, Kimm SY. Childhood obesity. Lancet. 2010;375:1737–48. doi: 10.1016/S0140-6736(10)60171-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.