Abstract
Aims
Acute kidney injury (AKI) is associated with a high hospitalization rate, accelerated long‐term decline in kidney function and a high mortality rate. Adverse drug reactions (ADRs) constitute one of the most important modifiable factors in the context of AKI. Most studies of drug‐induced AKI have focused on a sole drug class. The objective of the present study was to establish a comprehensive overview of drug‐induced AKI on the basis of spontaneously reported ADRs in the French national pharmacovigilance database (FPVD).
Methods
We performed a case–noncase study of drug‐induced AKI. Cases corresponded to the reports of AKI recorded in the FPVD between 1 January 2015 and 31 December 2015. The noncases corresponded to all other spontaneously reported ADRs (excluding AKI) recorded in the FPVD during the same period. Data were expressed as the reporting odds ratio (ROR) and the 95% confidence interval.
Results
Of the 38 782 ADRs recorded in the FPVD during the study period, 3.2% were classified as cases of AKI. A total of 1254 patients experienced AKI (males: 55%; mean age ± standard deviation: 68.7 ± 15.0 years). Overall, 15.2% of the patients required renal replacement therapy. Two or more concomitantly administered drugs were involved in 66% of the cases of AKI. The most frequently implicated drug classes were antibacterial agents for systemic use (29.5%), diuretics (18.5%), agents acting on the renin–angiotensin system (16.3%), antineoplastic agents (10.2%) and anti‐inflammatory agents (5.4%). Gentamicin, eplerenone, spironolactone, candesartan, cisplatin and acyclovir had the highest RORs (>10).
Conclusion
A comprehensive study of a national pharmacovigilance database enabled us to identify the drug classes that most frequently induced AKI. Even though most of the identified drugs were already known to induce AKI, the present work should raise physicians' awareness of the compounds responsible for triggering this potentially life‐threatening condition.
Keywords: acute kidney injury, drug, nephrotoxicity, pharmacovigilance database
What is Already Known about this Subject
Different drugs have been reported as inducing acute kidney injury (AKI). However, most studies of drug‐induced AKI have focused on a sole drug class, so we aimed to present a comprehensive overview of drug‐induced AKI.
What this Study Adds
We identified the drug classes that most frequently induced AKI. The present work should raise physicians' awareness of the compounds responsible for triggering this potentially life‐threatening condition.
Introduction
Acute kidney injury (AKI) is a common condition; it affects 15% of inpatients and up to 40–60% of patients in the intensive care unit 1, 2, 3. AKI is associated with longer hospital stays and a higher mortality rate 2, 3, 4, 5. The Kidney Disease Improving Global Outcomes (KDIGO) working group has published a definition of AKI 6. Regardless of the severity of injury, all patients with AKI are at risk of sequelae; acute damage may become irreversible damage, with progression to chronic kidney disease (CKD) and an increased risk of recurrence. After an episode of AKI, kidney function is presumed to have recovered fully if serum creatinine levels return to baseline. However, recent data show that up to 70% of elderly patients develop de novo CKD within 2 years of an episode of AKI 7. In view of the unpredictable prognosis and high healthcare costs associated with AKI, preventing this serious condition is a public health issue.
The main known risk factors for AKI include underlying kidney disease, diabetes, cardiovascular diseases and acute situations such as dehydration 8, 9. Adverse drug reactions (ADRs) are also a common cause of AKI. Many drugs can induce or aggravate the condition. Drug‐induced AKI accounts for 19% of cases of AKI in a hospital setting 9. Different mechanisms of action have been identified: a decrease in kidney perfusion pressure, cytotoxicity, hypersensitivity reactions and tubule obstruction by crystals. A single type of drug may even be responsible for several types of damage 8.
Although the role of exposure to these drugs is well understood, the drugs inducing AKI most frequently have not been clearly identified. It is important to identify AKI‐inducing drugs accurately because of the extent and severity of this disease and the large number of medications concerned. Few studies have sought specifically to identify the medications that are most frequently responsible for AKI, and most of the research has been restricted to elderly patient populations 10, 11. Furthermore, the validated method applied in the present study has never before been used in this field.
Hence, the objective of the present dataset analysis was to establish a comprehensive nationwide overview of drug‐induced AKI by studying spontaneously reported ADRs in the French national pharmacovigilance database (FPVD). Identifying the drugs most frequently implicated in the occurrence of an AKI could improve prescribing practice and strengthen monitoring and nephroprotective measures in patients at risk of developing an AKI.
Methods
The national pharmacovigilance system in France was established in 1973. It consists of a network of 31 regional centres to which ADRs should be reported spontaneously. The FPVD was established in 1985 to record any ADR notified spontaneously by health professionals. The present dataset analysis was approved by 31 regional pharmacovigilance centres and the French National Healthcare Products and Drug Safety Agency [Agence nationale de sécurité du médicament et des produits de santé (ANSM)], which manages the database and helped us with data extraction. It should be noted that the authors of the present article were solely responsible for interpretation of the data, and that the ANSM was not involved in the interpretation.
An ADR is categorized as ‘serious’ if it results in any untoward medical occurrence that (at any dose) results in death, requires initial or prolonged hospitalization, results in persistent or significant disability/incapacity, or is life threatening. For each reported ADR, information on the patient (e.g. age, gender), the reaction itself (e.g. date of occurrence, and progression) and drug exposure (e.g. dates of introduction and withdrawal) is recorded in the FPVD, along with a summary of the clinical report. The regional pharmacovigilance centres evaluate the causal relationships between adverse events and drugs. For each report, causality is assessed according to the French national pharmacovigilance system's standard procedure 12. If a drug is considered to be definitely or probably responsible for the adverse event, it is defined as ‘suspect’. If not, it is defined as ‘associated’. ADRs are coded according to the Medical Dictionary for Regulatory Activities (MedDRA version 18.1) 13.
In the present study, we selected ADRs recorded in the FPVD between 1 January 2015 and 31 December 2015. Cases were identified using the broad‐ranging ‘acute renal disease’ standardized MedDRA query (SMQ).
This broad SMQ was chosen so as not to omit cases of AKI coded under another MedDRA term (Table 1). We excluded adverse events in which the serum creatinine level did not exceed 1.5 times the age‐adjusted reference value (a criterion based on the Kidney Disease: Improving Global Outcomes classification 14). We also excluded adverse events concerning patients under the age of 18 years.
Table 1.
MedDRA terms included in the standardized MedDRA query ‘acute renal failure’ [20000003]
| Acute kidney injury | Creatinine renal clearance abnormal |
| Acute phosphate nephropathy | Creatinine renal clearance decreased |
| Acute prerenal failure | Creatinine urine abnormal |
| Anuria | Creatinine urine decreased |
| Azotaemia | Crystal nephropathy |
| Continuous haemodiafiltration | Fractional excretion of sodium |
| Dialysis | Glomerular filtration rate abnormal |
| Haemodialysis | Glomerular filtration rate decreased |
| Haemofiltration | Hypercreatininaemia |
| Neonatal anuria | Intradialytic parenteral nutrition |
| Nephropathy toxic | Nephritis |
| Oliguria | Oedema due to renal disease |
| Peritoneal dialysis | Protein urine present |
| Prerenal failure | Proteinuria |
| Renal failure | Renal function test abnormal |
| Renal failure neonatal | Renal transplant |
| Renal impairment | Renal tubular disorder |
| Albuminuria | Renal tubular necrosis |
| Blood creatinine abnormal | Tubulointerstitial nephritis |
| Blood creatinine increased | Urea renal clearance decreased |
| Blood urea abnormal | Urine output decreased |
| Blood urea increased | Blood urea nitrogen/creatinine ratio increased |
In the present case–non‐case study, the noncases corresponded to all spontaneously reported ADRs other than AKI recorded in the FPVD during the same period.
Analysed drugs
We analysed only drugs considered to be ‘suspect’ in at least five cases of AKI. This threshold was established to ensure that the data were normally distributed. The drugs were analysed according to their international nonproprietary name (INN). For drug combinations, each active substance was counted individually (except for sulfamethoxazole/trimethoprim). The Anatomical Therapeutic Chemical (ATC) classification was given for each drug. We also analysed the number of ‘ suspect drugs’ involved in each notified AKI and the outcome of the AKI.
Statistical analysis
The association between AKI and drug (INN) exposure was assessed as the reporting odds ratio (ROR) (as explained in Table 2) and its 95% confidence interval (CI), calculated using Woolf's method 15.
Table 2.
Calculation of the reporting odds ratio (ROR)
| Number of cases of AKI | Number of cases of other ADRs | |
|---|---|---|
| Studied drug | a | b |
| Other drugs | c | d |
ROR = (a/c)/(b/d)
a, number of exposed cases (AKI with the studied drug); b, number of exposed noncases (all ADRs other than AKI with the studied drug); c, number of non‐exposed cases (AKI with other drugs); d, number of unexposed noncases (all ADRs other than AKI with other drugs); ADR, adverse drug reaction; AKI, acute kidney injury.
Total number of ADRs reported in 2015 : a + b + c + d
Results
Of the 38 782 reports recorded in the FPVD between 1 January 2015, and 31 December 2015, 1568 matched the SMQ for ‘acute renal failure’. We excluded 87 events in patients under the age of 18 years and 227 in which the serum creatinine level did not exceed 1.5 times the age‐adjusted reference value. Hence, a total of 1254 cases of AKI were analysed (corresponded to 3.2% of all records in the FPVD in 2015) (Figure 1). Accordingly, there were 37 528 noncases.
Figure 1.
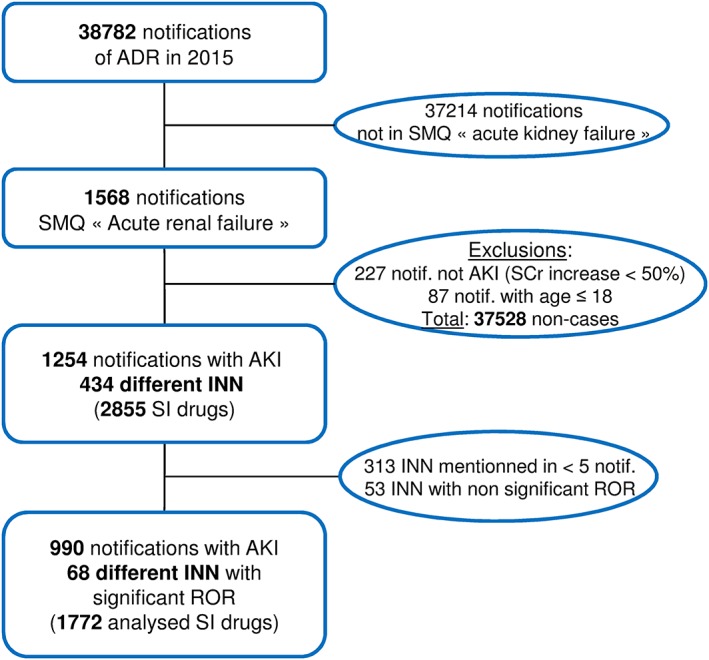
Study design. ADR, adverse drug reaction; AKI, acute kidney injury; INN, international nonproprietary name; ROR, reporting odds ratio; SCr, serum creatinine; SI: ‘suspect’ or ‘interacting’; SMQ, standardized MedDRA query
All analysed cases of AKI had been notified by healthcare professionals. Forty‐five per cent of the patients were female. The mean age ± standard deviation of patients with AKI was 68.7 ± 15 years. Figure 2 shows the age distribution of the study population. The AKI outcomes were distributed as follows: 4.6% of the patients died, 80.7% had recovered or were recovering, and the outcome was unknown for the remaining 14.7%. More than one drug was suspected in 66% of the AKIs (Figure 3). Overall, 15.2% of the patients required renal replacement therapy (RRT) (Table 3), and 44.0% of these cases were female. Gender was not associated with the need for RRT, although the patients requiring RRT were significantly younger than the patients not requiring RRT (66.6 ± 14.6 vs. 69.1 ± 15.8, respectively; P = 0.042). The involvement of two or more drugs was suspected in 61.3% of the cases of AKI in patients requiring RRT.
Figure 2.
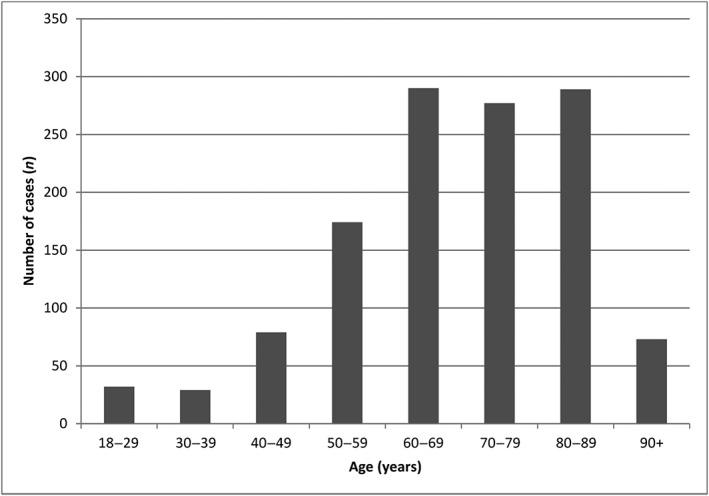
Distribution of the acute kidney injury events by age group
Figure 3.
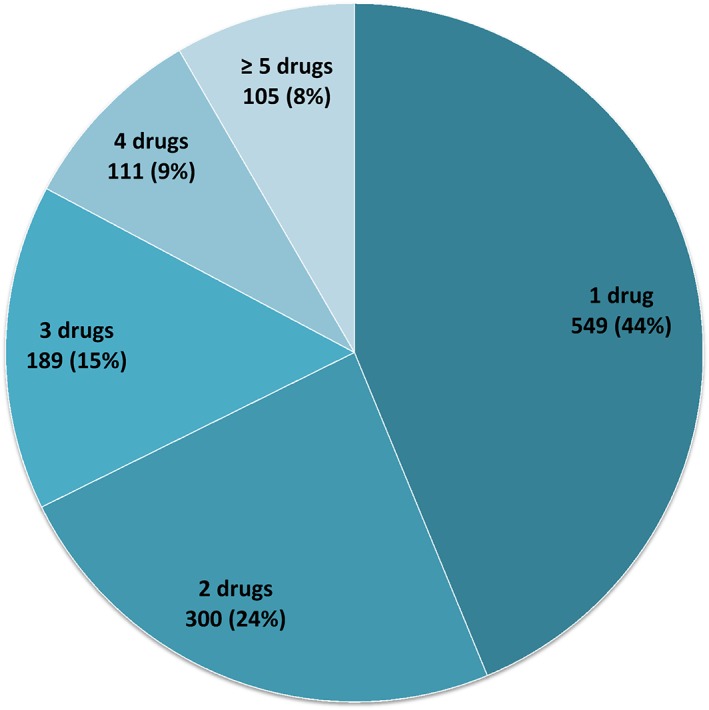
Distribution of the 1254 reported acute kidney injury events as a function of the number of ‘suspect’ drugs per event [number (%)]
Table 3.
Drugs with a statistically significant ROR and that were mentioned in at least five AKI events in the FPVD
| ATC classification | Active substance (INN) | Total number of ADRs with AKI n | Number of ADRs with AKI when the drug is suspected alone n (%) | Patients requiring RRT n (%) | Total number of ADRs without AKI n | ROR | [95%CI] |
|---|---|---|---|---|---|---|---|
| Antibacterial agents for systemic use and antimycobacterial agents | 523 | ||||||
| Amikacin | 24 | 8 (33.33) | 7 (29.17) | 78 | 9.37 | [5.91–14.86] | |
| Amoxicillin | 98 | 21 (21.43) | 15 (15.31) | 1617 | 1.88 | [1.52–2.33] | |
| Ceftriaxone | 26 | 1 (3.85) | 6 (23.08) | 481 | 1.63 | [1.09–2.43] | |
| Cilastatin | 8 | 0 (0.00) | 1 (12.5) | 108 | 2.22 | [1.08–4.57] | |
| Ciprofloxacin | 18 | 7 (38.89) | 4 (22.22) | 279 | 1.94 | [1.2–3.14] | |
| Cloxacillin | 24 | 9 (37.50) | 1 (4.17) | 120 | 6.08 | [3.91–9.46] | |
| Daptomycin | 7 | 2 (28.57) | 0 (0.00) | 57 | 3.69 | [1.68–8.11] | |
| Gentamicin | 53 | 6 (11.32) | 15 (28.3) | 89 | 18.56 | [13.15–26.21] | |
| Imipenem | 8 | 0 (0.00) | 1 (12.5) | 115 | 2.09 | [1.02–4.29] | |
| Ofloxacin | 16 | 3 (18.75) | 1 (6.25) | 286 | 1.68 | [1.01–2.79] | |
| Piperacillin | 32 | 0 (0.00) | 3 (9.38) | 429 | 2.26 | [1.57–3.26] | |
| Sulfadiazine | 5 | 4 (80) | 0 (0.00) | 42 | 3.57 | [1.41–9.05] | |
| Sulfamethoxazole and trimethoprim | 73 | 29 (39.73) | 3 (4.11) | 548 | 4.17 | [3.25–5.36] | |
| Tazobactam | 32 | 0 (0.00) | 3 (9.38) | 412 | 2.36 | [1.64–3.4] | |
| Teicoplanin | 6 | 2 (33.33) | 0 (0.00) | 55 | 3.28 | [1.41–7.62] | |
| Vancomycin | 67 | 19 (28.36) | 11 (16.42) | 304 | 6.91 | [5.27–9.06] | |
| Rifampicin | 26 | 4 (15.38) | 1 (3.85) | 325 | 2.42 | [1.62–3.63] | |
| Diuretics | 328 | ||||||
| Altizide | 5 | 0 (0.00) | 0 (0.00) | 29 | 5.18 | [2–13.4] | |
| Eplerenone | 10 | 1 (10) | 0 (0.00) | 11 | 27.42 | [11.62–64.68] | |
| Furosemide | 172 | 20 (11.63) | 21 (12.21) | 358 | 16.5 | [13.63–19.99] | |
| Hydrochlorothiazide | 88 | 3 (3.41) | 9 (10.23) | 438 | 6.39 | [5.05–8.09] | |
| Indapamide | 11 | 0 (0.00) | 2 (18.18) | 140 | 2.36 | [1.28–4.38] | |
| Spironolactone | 42 | 2 (4.76) | 4 (9.52) | 123 | 10.54 | [7.39–15.03] | |
| Agents acting on the renin‐angiotensin system | 289 | ||||||
| Candesartan | 33 | 2 (6.06) | 10 (30.3) | 93 | 10.88 | [7.28–16.25] | |
| Enalapril | 12 | 1 (8.33) | 1 (8.33) | 86 | 4.21 | [2.29–7.72] | |
| Irbesartan | 22 | 3 (13.64) | 3 (13.64) | 167 | 3.99 | [2.55–6.25] | |
| Lisinopril | 7 | 1 (14.29) | 0 (0.00) | 30 | 7.02 | [3.08–16.01] | |
| Losartan | 10 | 1 (10.00) | 2 (20.00) | 41 | 7.35 | [3.67–14.71] | |
| OlmesartanMedoxomil | 49 | 6 (12.24) | 8 (16.33) | 201 | 7.55 | [5.5–10.37] | |
| Perindopril | 72 | 10 (13.89) | 8 (11.11) | 326 | 6.95 | [5.35–9.03] | |
| Ramipril | 49 | 11 (22.45) | 8 (16.33) | 261 | 5.81 | [4.26–7.92] | |
| Telmisartan | 5 | 0 (0.00) | 0 (0.00) | 39 | 3.85 | [1.51–9.78] | |
| Valsartan | 30 | 0 (0.00) | 3 (10.00) | 144 | 6.36 | [4.28–9.47] | |
| Antineoplastic agents | 180 | ||||||
| Bendamustine | 20 | 7 (35.00) | 2 (10.00) | 66 | 9.2 | [5.56–15.22] | |
| Cisplatin | 44 | 20 (45.45) | 8 (18.18) | 130 | 10.46 | [7.4–14.79] | |
| Cytarabine | 17 | 1 (5.88) | 5 (29.41) | 157 | 3.27 | [1.98–5.41] | |
| Etoposide | 12 | 0 (0.00) | 2 (16.67) | 175 | 2.06 | [1.15–3.71] | |
| Gemcitabine | 13 | 4 (30.77) | 4 (30.77) | 131 | 2.99 | [1.69–5.3] | |
| Melphalan | 8 | 1 (12.50) | 2 (25.00) | 59 | 4.08 | [1.94–8.55] | |
| Methotrexate | 39 | 20 (51.28) | 8 (20.51) | 553 | 2.15 | [1.54–2.98] | |
| Nivolumab | 7 | 6 (85.71) | 2 (28.57) | 90 | 2.34 | [1.08–5.05] | |
| Pemetrexed | 13 | 2 (15.38) | 2 (15.38) | 91 | 4.31 | [2.4–7.73] | |
| Vemurafenib | 7 | 3 (42.86) | 1 (14.29) | 64 | 3.29 | [1.5–7.18] | |
| Anti‐inflammatory and antirheumatic products | 95 | ||||||
| Diclofenac | 33 | 12 (36.36) | 4 (12.12) | 161 | 6.27 | [4.29–9.16] | |
| Ibuprofen | 25 | 8 (32.00) | 2 (8.00) | 373 | 2.03 | [1.35–3.05] | |
| Ketoprofen | 26 | 6 (23.08) | 5 (19.23) | 302 | 2.61 | [1.74–3.91] | |
| Naproxen | 11 | 4 (36.36) | 3 (27.27) | 90 | 3.68 | [1.96–6.9] | |
| Drugs used in diabetes | 75 | ||||||
| Gliclazide | 8 | 0 (0.00) | 1 (12.50) | 76 | 3.16 | [1.52–6.57] | |
| Metformin | 58 | 17 (29.31) | 19 (32.76) | 349 | 5.17 | [3.89–6.86] | |
| Sitagliptin | 9 | 1 (11.11) | 1 (11.11) | 121 | 2.23 | [1.13–4.41] | |
| Antivirals for systemic use | 51 | ||||||
| Aciclovir | 37 | 21 (56.76) | 3 (8.11) | 49 | 23.25 | [15.12–35.77] | |
| Valaciclovir | 14 | 5 (35.71) | 2 (14.29) | 97 | 4.36 | [2.48–7.65] | |
| Calcium channel blockers | 41 | ||||||
| Amlodipine | 35 | 0 (0.00) | 7 (20.00) | 328 | 3.26 | [2.29–4.64] | |
| Nicardipine | 6 | 0 (0.00) | 3 (50.00) | 71 | 2.54 | [1.1–5.85] | |
| Immunosuppressants | 38 | ||||||
| Ciclosporin | 20 | 6 (30.00) | 5 (25.00) | 105 | 5.78 | [3.57–9.35] | |
| Everolimus | 7 | 0 (0.00) | 2 (28.57) | 93 | 2.26 | [1.05–4.88] | |
| Tacrolimus | 11 | 3 (27.27) | 1 (9.09) | 128 | 2.59 | [1.39–4.8] | |
| Contrast media | 32 | ||||||
| Iobitridol | 10 | 4 (40.00) | 2 (20.00) | 79 | 3.81 | [1.97–7.37] | |
| Iohexol | 16 | 5 (31.25) | 2 (12.5) | 74 | 6.54 | [3.8–11.26] | |
| Iopromide | 6 | 2 (33.33) | 3 (50.00) | 61 | 2.95 | [1.27–6.84] | |
| Miscellaneous drugs | 120 | ||||||
| Fenofibrate | 6 | 0 (0.00) | 3 (50.00) | 72 | 2.5 | [1.09–5.76] | |
| Atropine | 5 | 0 (0.00) | 1 (20.00) | 20 | 7.51 | [2.81–20.04] | |
| Bisoprolol | 17 | 0 (0.00) | 4 (23.53) | 265 | 1.93 | [1.18–3.17] | |
| Immunoglobulins | 23 | 7 (30.43) | 15 (65.22) | 361 | 1.92 | [1.26–2.94] | |
| Colchicine | 37 | 12 (32.43) | 5 (13.51) | 155 | 7.33 | [5.1–10.54] | |
| Zoledronic acid | 10 | 9 (90.00) | 2 (20.00) | 103 | 2.92 | [1.52–5.6] | |
| Sevoflurane | 5 | 1 (20.00) | 0 (0.00) | 9 | 16.69 | [5.58–49.87] | |
| Lithium | 17 | 11 (64.71) | 3 (17.65) | 240 | 2.14 | [1.3–3.5] | |
ADR, adverse drug reaction; ATC, Anatomical, Therapeutic and Chemical; AKI, acute kidney injury; CI, confidence interval; FPVD, French national pharmacovigilance database; INN, international nonproprietary name; ROR, reporting odds ratio; RRT, renal replacement therapy
In all, 434 different drugs were considered as being ‘suspect’ in cases of AKI. Of these, 121 drugs were mentioned in at least five notifications. The 68 drugs with a statistically significant ROR are shown in Table 3. We also calculated the number of cases of AKI in which each drug was the only ‘suspect’; the 68 drugs were mentioned 1772 times in 2015.
According to the ATC classification, antibacterial agents for systemic use were the most frequently reported drugs (n = 528; 29.5%); amoxicillin and sulfamethoxazole/trimethoprim were reported 98 (5.5%) and 73 (4.1%) times, respectively. Diuretics were reported 328 times (18.5%) and agents acting on the renin–angiotensin system were reported 289 times (16.3%). Within the latter class, angiotensin II antagonists (ARBs) were reported 149 times (8.4%) and angiotensin‐converting enzyme inhibitors (ACEIs) were reported 140 times (7.9%). Lastly, antineoplastic agents were reported 180 times (10.2%), and anti‐inflammatory and anti‐rheumatic drugs were reported 95 (5.4%) times.
Discussion
In a large, nationwide study of recent pharmacovigilance data, we obtained a comprehensive overview of the risk of drug‐induced AKI. Of the 38 782 ADR reports recorded in the FPVD during the study period, 1254 (3.2%) corresponded to AKI. The mean age of the patients having developed AKI was 68.7 ± 15.0 years. Elderly patients often have multiple risk factors for AKI, such as concomitant diseases (e.g. cardiovascular disease, CKD, diabetes and heart failure), dehydration, hypotension, increased exposure to diagnostic procedures involving radiocontrast agents, and the administration of one or more nephrotoxic medications. Indeed, Feest et al. 10 showed that there is a progressive, age‐dependent, three‐ to eight‐fold increase in the incidence of community‐acquired AKI in patients over the age of 60 years. Groeneveld et al. 11 showed that the age‐related yearly incidence of AKI rose from 17 per million in adults under 50 years to 949 per million in the 80–89‐year age group.
In line with the data in the literature, 616 notifications (62.2%) in the present analysis involved two or more ‘suspect’ drugs. The risk of AKI induction is particularly high in the context of polypharmacy, and increases with the number of drugs prescribed. In one study, it was shown that the simultaneous administration of at least two cardiovascular drugs (antihypertensive agents, antiarrhythmic agents, diuretics, platelet aggregation inhibitors, lipid‐lowering agents and digoxin) was significantly associated with an increased risk of AKI 16. Similarly, a study of the large Taiwan National Health Insurance Research Database found that polypharmacy for 31–90 days, 91–180 days and over 181 days was respectively associated with odds ratios for developing acute kidney failure of 1.33 (P < 0.001), 1.65 (P < 0.001) and 1.74 (P < 0.001), when compared with polypharmacy for less than 30 days 17.
In the present study, the drug classes most frequently associated with AKI were antibacterial agents for systemic use, diuretics, agents acting on the renin–angiotensin system, antineoplastic agents and nonsteroidal anti‐inflammatory drugs (NSAIDs). In a previous study of unplanned hospitalizations caused by ADRs in older US war veterans, antibiotics, loop diuretics, ACEIs and NSAIDs were identified as the main pharmacological classes responsible 18. More recently, Handler et al. found that the vast majority of cases of drug‐associated AKI in nursing home residents were related to the use of diuretics, ACEIs, ARBs and antibiotics 19.
Our results were consistent with the data in the current literature: 60 of the 68 analysed drugs were known to cause kidney damage (i.e. as described in the Summary of Product Characteristics or in the Micromedex drug information database) 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40.
The main strength of the present study was its exhaustive, nationwide analysis of all drug‐induced episodes of AKI over a 12‐month period. It is noteworthy than many of the affected patients were elderly and were taking several potentially nephrotoxic drugs. The most frequently implicated drug classes included some very frequently prescribed medications. These drugs are used to treat acute and chronic diseases, some of which can also promote the occurrence of AKI (e.g. heart failure, hypertension, diabetes, infectious diseases and cancer). In addition, this was the first time that disproportionality analysis (a previously validated method 41, 42) has been applied to the assessment of drug‐induced AKI. All the required information was present in the database and readily available for analysis. Access to the FPVD is restricted to the 31 regional pharmacovigilance centres in France (as part of a national network established by the ANSM).
The present analysis combined the limitations of case–control methods with those of spontaneous reporting. As in all pharmacovigilance studies, we were unable to calculate the true incidence of the ADRs – notably because of underreporting. However, the magnitude of underreporting does not affect the results of this kind of study 42. Furthermore, the objective of the present study was not to calculate the true incidence of drug‐induced AKI or to provide an exhaustive description of all cases of drug‐induced AKI. In this kind of study, the ROR corresponds to the risk of spontaneous notification of an ADR and not the risk of AKI occurrence per se. The ROR may also be artificially decreased if another drug‐specific reaction is reported more consistently; this dilutes the association by increasing the frequency of the drug among the noncase reports. For example, vitamin K antagonists are well known to cause interstitial nephritis but were not associated with a statistically significant ROR in the present dataset analysis. This might be because the ADR most frequently reported to the pharmacovigilance network for this drug class is bleeding – resulting in a very small proportion of AKI events. Lastly, data in the FPVD on the patient's medical history (including CKD and diabetes) were sparse, which prevented us from analysing other risk factors possibly associated with AKI. The present method did not provide us with detailed information on the patients' clinical status. Clinically unstable patients are more likely to develop AKI and to be taking several concomitant drugs than stable patients; this may be a confounding factor in the occurrence of AKI.
Conclusion
In the present large‐scale study of a nationwide pharmacovigilance database, we identified the main drug classes implicated in AKI by applying a validated method (disproportionality analysis) to this field for the first time. Even though most of the identified drugs were already known to induce AKI, our present results should raise physicians' awareness of the drugs responsible for inducing AKI. Knowledge of a drug's mechanism of action may help to anticipate (and thus avoid) renal ADRs. When an at‐risk situation is identified (hypovolaemia, shock, etc.), withdrawal of nephrotoxic treatments should be considered, lessening the severity or even preventing the event from occurring. This requires highly responsive monitoring by prescribers and better education for patients treated with these drugs.
Competing Interest
There are no competing interests to declare.
Contributors
M.P.M., V.G., J.M. and S.L. contributed to the conception/design of the work; the acquisition, analysis and interpretation of the data; drafting the work and revising it critically for important intellectual content. K.M., G.C., J.M., L.G. and S.P. revised the article critically for important intellectual content.
Pierson‐Marchandise, M. , Gras, V. , Moragny, J. , Micallef, J. , Gaboriau, L. , Picard, S. , Choukroun, G. , Masmoudi, K. , Liabeuf, S. , and the French National Network of Pharmacovigilance Centres (2017) The drugs that mostly frequently induce acute kidney injury: a case − noncase study of a pharmacovigilance database. Br J Clin Pharmacol, 83: 1341–1349. doi: 10.1111/bcp.13216.
References
- 1. Mehta RL, Pascual MT, Soroko S, Savage BR, Himmelfarb J, Ikizler TA, et al. Spectrum of acute renal failure in the intensive care unit: the PICARD experience. Kidney Int 2004; 66: 1613–1621. [DOI] [PubMed] [Google Scholar]
- 2. Ali T, Khan I, Simpson W, Prescott G, Townend J, Smith W, et al. Incidence and outcomes in acute kidney injury: a comprehensive population‐based study. J Am Soc Nephrol 2007; 18: 1292–1298. [DOI] [PubMed] [Google Scholar]
- 3. Hoste EAJ, Bagshaw SM, Bellomo R, Cely CM, Colman R, Cruz DN, et al. Epidemiology of acute kidney injury in critically ill patients: the multinational AKI‐EPI study. Intensive Care Med 2015; 41: 1411–1423. [DOI] [PubMed] [Google Scholar]
- 4. Coca SG, Singanamala S, Parikh CR. Chronic kidney disease after acute kidney injury: a systematic review and meta‐analysis. Kidney Int 2012; 81: 442–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Xue JL, Daniels F, Star RA, Kimmel PL, Eggers PW, Molitoris BA, et al. Incidence and mortality of acute renal failure in Medicare beneficiaries, 1992 to 2001. J Am Soc Nephrol 2006; 17: 1135–1142. [DOI] [PubMed] [Google Scholar]
- 6. Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract 2012; 120: c179–c184. [DOI] [PubMed] [Google Scholar]
- 7. Triverio P‐A, Martin P‐Y, Romand J, Pugin J, Perneger T, Saudan P. Long‐term prognosis after acute kidney injury requiring renal replacement therapy. Nephrol Dial Transplant 2009; 24: 2186–2189. [DOI] [PubMed] [Google Scholar]
- 8. Perazella MA. Renal vulnerability to drug toxicity. Clin J Am Soc Nephrol 2009; 4: 1275–1283. [DOI] [PubMed] [Google Scholar]
- 9. Uchino S, Kellum JA, Bellomo R, Doig GS, Morimatsu H, Morgera S, et al. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA 2005; 294: 813–818. [DOI] [PubMed] [Google Scholar]
- 10. Feest TG, Round A, Hamad S. Incidence of severe acute renal failure in adults: results of a community based study. BMJ (Clinical research ed) 1993; 306: 481–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Groeneveld AB, Tran DD, Meulen J, Nauta JJ, Thijs LG. Acute renal failure in the medical intensive care unit: predisposing, complicating factors and outcome. Nephron 1991; 59: 602–610. [DOI] [PubMed] [Google Scholar]
- 12. Bégaud B, Evreux JC, Jouglard J, Lagier G. Imputation of the unexpected or toxic effects of drugs. Actualization of the method used in France. Therapie 1985; 40: 111–118. [PubMed] [Google Scholar]
- 13. Brown EG, Wood L, Wood S. The medical dictionary for regulatory activities (MedDRA). Drug Saf 1999; 20: 109–117. [DOI] [PubMed] [Google Scholar]
- 14. Okusa MD, Davenport A. Reading between the (guide)lines‐‐the KDIGO practice guideline on acute kidney injury in the individual patient. Kidney Int 2014; 85: 39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Woolf B. On estimating the relation between blood group and disease. Ann Hum Genet 1955; 19: 251–253. [DOI] [PubMed] [Google Scholar]
- 16. Chao CT, Tsai HB, Wu CY, Lin YF, Hsu NC, Chen JS, et al. Cumulative cardiovascular polypharmacy is associated with the risk of acute kidney injury in elderly patients. Medicine 2015; 94: e1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chang Y‐P, Huang S‐K, Tao P, Chien C‐W. A population‐based study on the association between acute renal failure (ARF) and the duration of polypharmacy. BMC Nephrol 2012; 13: 96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Marcum ZA, Amuan ME, Hanlon JT, Aspinall SL, Handler SM, Ruby CM, et al. Prevalence of unplanned hospitalizations caused by adverse drug reactions in older veterans. J Am Geriatr Soc 2012; 60: 34–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Handler SM, Cheung PW, Culley CM, Perera S, Kane‐Gill SL, Kellum JA, et al. Determining the incidence of drug‐associated acute kidney injury in nursing home residents. J Am Med Dir Assoc 2014; 15: 719–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Walker RJ, Bailey RR, Lynn KL, Swainson CP. Amoxycillin‐induced acute interstitial nephritis. N Z Med J 1985; 98: 866. [PubMed] [Google Scholar]
- 21. Soto J, Bosch JM, Alsar Ortiz MJ, Moreno MJ, Gonzalez JD, Diaz JM. Piperacillin‐induced acute interstitial nephritis. Nephron 1993; 65: 154–155. [DOI] [PubMed] [Google Scholar]
- 22. Bakker SJ, Luik AJ, Leunissen KM. Flucloxacillin‐induced acute interstitial nephritis. Nephrol Dial Transplant 1995; 10: 579. [DOI] [PubMed] [Google Scholar]
- 23. Wai AO, Lo AM, Abdo A, Marra F. Vancomycin‐induced acute interstitial nephritis. Ann Pharmacother 1998; 32: 1160–1164. [DOI] [PubMed] [Google Scholar]
- 24. Kahlmeter G, Dahlager JI. Aminoglycoside toxicity – a review of clinical studies published between 1975 and 1982. J Antimicrob Chemother 1984; 13 (Suppl. A): 9–22. [DOI] [PubMed] [Google Scholar]
- 25. Lopez‐Novoa JM, Quiros Y, Vicente L, Morales AI, Lopez‐Hernandez FJ. New insights into the mechanism of aminoglycoside nephrotoxicity: an integrative point of view. Kidney Int 2011; 79: 33–45. [DOI] [PubMed] [Google Scholar]
- 26. Fraser TN, Avellaneda AA, Graviss EA, Musher DM. Acute kidney injury associated with trimethoprim/sulfamethoxazole. J Antimicrob Chemother 2012; 67: 1271–1277. [DOI] [PubMed] [Google Scholar]
- 27. Bald M, Ratjen F, Nikolaizik W, Wingen AM. Ciprofloxacin‐induced acute renal failure in a patient with cystic fibrosis. Pediatr Infect Dis J 2001; 20: 320–321. [DOI] [PubMed] [Google Scholar]
- 28. Chiba S, Tsuchiya K, Sakashita H, Ito E, Inase N. Rifampicin‐induced acute kidney injury during the initial treatment for pulmonary tuberculosis: a case report and literature review. Internal Medicine (Tokyo, Japan) 2013; 52: 2457–2460. [DOI] [PubMed] [Google Scholar]
- 29. Mauri JM, Fort J, Bartolome J, Camps J, Capdevila L, Morlans M. Antirifampicin antibodies in acute rifampicin‐associated renal failure. Nephron 1982; 31: 177–179. [DOI] [PubMed] [Google Scholar]
- 30. Giustina A, Romanelli G, Cimino A, Brunori G. Low‐dose acyclovir and acute renal failure. Ann Intern Med 1988; 108: 312. [DOI] [PubMed] [Google Scholar]
- 31. Becker BN, Fall P, Hall C, Milam D, Leonard J, Glick A. Rapidly progressive acute renal failure due to acyclovir: case report and review of the literature. Am J Kidney Dis 1993; 22: 611–615. [DOI] [PubMed] [Google Scholar]
- 32. Naughton CA. Drug‐induced nephrotoxicity. Am Fam Physician 2008; 78: 743–750. [PubMed] [Google Scholar]
- 33. Tomlinson LA, Abel GA, Chaudhry AN, Tomson CR, Wilkinson IB, Roland MO, et al. ACE inhibitor and angiotensin receptor‐II antagonist prescribing and hospital admissions with acute kidney injury: a longitudinal ecological study. PLoS One 2013; 8: e78465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Whelton A. Nephrotoxicity of nonsteroidal anti‐inflammatory drugs: physiologic foundations and clinical implications. Am J Med 1999; 106: 13S–24S. [DOI] [PubMed] [Google Scholar]
- 35. Clive DM, Stoff JS. Renal syndromes associated with nonsteroidal antiinflammatory drugs. N Engl J Med 1984; 310: 563–572. [DOI] [PubMed] [Google Scholar]
- 36. Lapi F, Azoulay L, Yin H, Nessim SJ, Suissa S. Concurrent use of diuretics, angiotensin converting enzyme inhibitors, and angiotensin receptor blockers with non‐steroidal anti‐inflammatory drugs and risk of acute kidney injury: nested case–control study. BMJ (Clinical research ed) 2013; 346: e8525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Arany I, Safirstein RL. Cisplatin nephrotoxicity. Semin Nephrol 2003; 23: 460–464. [DOI] [PubMed] [Google Scholar]
- 38. Widemann BC, Adamson PC. Understanding and managing methotrexate nephrotoxicity. Oncologist 2006; 11: 694–703. [DOI] [PubMed] [Google Scholar]
- 39. Johnson F, Phillips D, Talabani B, Wonnacott A, Meran S, Phillips AO. The impact of acute kidney injury in diabetes mellitus: AKI and diabetes mellitus. Nephrology 2016; 21: 506–511. [DOI] [PubMed] [Google Scholar]
- 40. Bartholomew BA B, Harjai KJ, Dukkipati S, Boura JA, Yerkey MW, Glazier S, et al. Impact of nephropathy after percutaneous coronary intervention and a method for risk stratification. Am J Cardiol 2004; 93: 1515–1519. [DOI] [PubMed] [Google Scholar]
- 41. Egberts ACG, Meyboom RHB, van Puijenbroek EP. Use of measures of disproportionality in pharmacovigilance: three Dutch examples. Drug Saf 2002; 25: 453–458. [DOI] [PubMed] [Google Scholar]
- 42. Montastruc J‐L, Sommet A, Bagheri H, Lapeyre‐Mestre M. Benefits and strengths of the disproportionality analysis for identification of adverse drug reactions in a pharmacovigilance database. Br J Clin Pharmacol 2011; 72: 905–908. [DOI] [PMC free article] [PubMed] [Google Scholar]


