Abstract
LINKED ARTICLE
This article is commented on by Bateman DN and Dear JW. Should we treat very large paracetamol overdose differently? Br J Clin Pharmacol 2017; 83: 1163–5. https://doi.org/10.1111/bcp.13279
Aims
Treatment of paracetamol (acetaminophen) overdose with acetylcysteine is standardized, with dose determined only by patient weight. The validity of this approach for massive overdoses has been questioned. We systematically compared outcomes in massive and non‐massive overdoses, to guide whether alternative treatment strategies should be considered, and whether the ratio between measured timed paracetamol concentrations (APAPpl) and treatment nomogram thresholds at those time points (APAPt) provides a useful assessment tool.
Methods
This is a retrospective observational study of all patients (n = 545) between 2005 and 2013 admitted to a tertiary care toxicology service with acute non‐staggered paracetamol overdose. Massive overdoses were defined as extrapolated 4‐h plasma paracetamol concentrations >250 mg l−1, or reported ingestions ≥30 g. Outcomes (liver injury, coagulopathy and kidney injury) were assessed in relation to reported dose and APAPpl:APAPt ratio (based on a treatment line through 100 mg l−1 at 4 h), and time to acetylcysteine.
Results
Ingestions of ≥30 g paracetamol correlated with higher peak serum aminotransferase (r = 0.212, P < 0.0001) and creatinine (r = 0.138, P = 0.002) concentrations. Acute liver injury, hepatotoxicity and coagulopathy were more frequent with APAPpl:APAPt ≥ 3 with odds ratios (OR) and 95% confidence intervals (CI) of 9.19 (5.04–16.68), 35.95 (8.80–158.1) and 8.34 (4.43–15.84), respectively (P < 0.0001). Heightened risk persisted in patients receiving acetylcysteine within 8 h of overdose.
Conclusion
Patients presenting following massive paracetamol overdose are at higher risk of organ injury, even when acetylcysteine is administered early. Enhanced therapeutic strategies should be considered in those who have an APAPpl:APAPt ≥ 3. Novel biomarkers of incipient liver injury and abbreviated acetylcysteine regimens require validation in this patient cohort.
Keywords: acetylcysteine, coagulopathy, hepatotoxicity, overdose, paracetamol
What is Already Known about this Subject
Acetylcysteine protocols in paracetamol overdose were initially developed empirically, with subsequent validation using pharmacokinetic studies of non‐toxic doses in healthy individuals.
It is unclear whether these modelling assumptions are robust in massive overdoses.
Case reports suggest that such patients may have worse outcomes, and biochemical data hint at the need for supplemental acetylcysteine.
What this Study Adds
Patients with an APAPpl:APAPt ≥ 3 (based on a treatment line through 100 mg l−1 at 4 h) have higher rates of organ injury.
Excess risk persists even with acetylcysteine administration within 8 h of overdose.
Patients with massive overdoses may benefit from higher or protracted doses of acetylcysteine, or approaches to enhance gastrointestinal drug elimination.
Table of Links
| LIGANDS |
|---|
| paracetamol |
This Table lists key ligands in this article that are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY 1.
Introduction
Paracetamol overdose remains the commonest drug overdose, and cause of acute liver failure, in Europe, Australia and North America 2, 3. Intravenous acetylcysteine is the mainstay of treatment and an effective antidote if used early in the course of poisoning 3, 4. The decision to treat acute, non‐staggered, paracetamol overdose is based principally on measured plasma paracetamol concentrations, taken at least 4 h after ingestion 3. International guidelines differ in their recommendations as to threshold paracetamol concentrations for treatment on nomograms, but once these have been exceeded, acetylcysteine dosing regimens are very similar throughout the world 5. The dose of acetylcysteine is determined only by patient weight, and does not vary according to other factors including the dose of paracetamol taken, plasma paracetamol concentration, time to presentation, and/or co‐ingestion of other drugs.
Acetylcysteine regimens have never been subject to definitive dose‐ranging studies in humans, nor have different regimens been compared in randomized controlled trials sufficiently powered to inform on the optimal strategy for preventing hepatotoxicity. As a result, current guidelines still advocate treatment based principally on the dose calculated in the 1970s for the initial studies of acetylcysteine in paracetamol toxicity 4. At that time, there were few data to inform on appropriate dosing, and much of the initial work was empirical. It had, however, been established that hepatic and renal toxicity were mediated through formation of N‐acetyl‐p‐benzoquinone imine (NAPQI), once paracetamol conjugation through glucuronidation and sulphation had been saturated 6. NAPQI can be detoxified to cysteine and mercapturate conjugates by glutathione, with organ injury resulting once stores of the latter become depleted 7. Consequently, pharmacokinetic studies were performed in healthy individuals to determine the level of glutathione depletion over a range of paracetamol concentrations, and a dose of acetylcysteine selected that would match this on a stoichiometric basis 5, 7.
Whilst this standard treatment regimen has proven extremely successful, the ‘one size fits all’ approach has been criticized 5, 8, 9. In particular, it is not clear whether the modelling assumptions underlying the initial acetylcysteine dose calculations hold true with very large overdoses, and whether therapy could be better tailored to individual cases in these situations 10, 11. Several case reports, and one recent observational study, highlight adverse outcomes in patients with massive paracetamol overdoses despite early acetylcysteine treatment 12, 13, 14, 15, 16. Such patients have higher cysteine and mercapturate to glucuronide conjugate ratios, implying increased proportions of paracetamol undergoing conversion to NAPQI and consistent with the need for supplemental acetylcysteine beyond that suggested by the original models 6. The aims of this study were to evaluate the development of organ injury in massive overdoses in a systematic manner, compare this to non‐massive ingestions, and assess whether reported ingested dose or the ratio of the measured plasma paracetamol concentration (APAPpl) to the corresponding treatment nomogram paracetamol concentration threshold at that time (APAPt) provided superior prediction of outcome. We assessed all patients presenting to a specialist toxicology service with acute paracetamol overdose meeting criteria for treatment with acetylcysteine, and determined outcomes for those taking massive overdoses.
Methods
Patients and clinical data
Clinical data on all patients presenting to our large inner‐city hospital with toxicology‐related problems are prospectively entered into a purpose‐designed clinical database, with follow‐up to the end of the acute inpatient admission episode 17. Data were extracted for all individuals who had taken an acute, single (non‐staggered) overdose of paracetamol in whom the time of ingestion was recorded, and who received treatment with acetylcysteine, between May 2005 and May 2013. There are no universally agreed criteria of what constitutes a massive paracetamol overdose; we therefore defined this pragmatically as an extrapolated 4‐h plasma paracetamol concentration > 250 mg l−1 (2.5‐fold the current UK threshold requiring treatment with acetylcysteine), or (where plasma paracetamol concentrations were not available) if a patient reported ingestion of ≥30 g paracetamol. Caldicott and Ethical Approval are in place for the database; data for this study were analysed anonymously and therefore no further ethical approval was required. This manuscript is written in compliance with STROBE guidelines.
The following information was extracted from the database: basic demographic data; time of presentation to the Emergency Department; reported quantity and time of paracetamol ingested; plasma paracetamol concentration (APAPpl) and time (t) this blood test was performed relative to exposure; time to initiation of acetylcysteine; and peak serum aminotransferase concentration, international normalized ratio (INR) and serum creatinine occurring on admission or during the course of treatment. Calculated 4‐h plasma paracetamol concentrations were back‐extrapolated from measured values using the formula used in previous studies: APAPpl/2e−(0.693/4)t 18. We also calculated the ratio between APAPpl and the threshold concentration at that time point on the treatment nomogram (based on a line through 100 mg l−1 at 4 h) above which acetylcysteine would be administered (APAPt).
Treatment regimens during study period
In the UK prior to 2012, single (non‐staggered) paracetamol overdoses were treated with acetylcysteine if measured plasma paracetamol concentrations were above a nomogram line starting at 200 mg l−1 at 4 h if deemed standard risk, or 100 mg l−1 at 4 h if high risk (e.g., patients with chronic alcohol misuse, medical conditions associated with glutathione depletion, and/or taking cytochrome P450‐2E1‐inducing medication). The standard acetylcysteine protocol was 150 mg kg−1 over 15 min, followed by 50 mg kg−1 over 4 h, and finally 100 mg kg−1 over 16 h. From 2012, UK guidelines changed such that everyone was treated according to the 100 mg l−1 at 4 h nomogram threshold line, with the duration of the first dose of acetylcysteine extended to 1 h 19. With both of these regimens, after completion of the third infusion, renal function, liver function and coagulation parameters are rechecked, and a further 16 h acetylcysteine infusion instituted in the event of any significant derangement 20.
Assessment of organ injury
There are a number of different working definitions for liver injury based on rises in serum alanine or aspartate aminotransferase concentrations, and data for all of the following were considered: (1) paracetamol‐related liver injury, defined by a ≥2‐fold rise above the upper limit of normal (ULN; the threshold above which UK guidelines recommend extending the acetylcysteine course) 8; (2) drug‐induced acute liver injury, defined as ≥3‐fold ULN 16, 21; and (3) paracetamol‐related hepatotoxicity, with aminotransferase concentrations >1000 IU l−1 4, 15, 22. Coagulopathy was defined as an INR rising above 1.3 (the threshold that would prompt extension of acetylcysteine therapy) 23, and significant acute kidney injury as a serum creatinine >150 μmol l−1 (in the absence of pre‐existing chronic kidney disease) 24. In addition, current UK guidelines advocate consideration for liver transplantation in paracetamol overdoses with an INR >6.5 or serum creatinine >300 μmol l−1 23.
Statistical analysis
Data are expressed as median (interquartile range), unless otherwise stated, and were analysed using GraphPad Prism (version 7.0; GraphPad Software, CA, USA, 2016). All eligible patients within the specified time frame were included in the study, and no formal power calculation was performed. Continuous variables were compared using the Mann–Whitney U‐test, correlation by Spearman rank coefficient, and event frequencies by Fisher's exact test. A P‐value of ≤0.05 was considered significant. Analyses did not impute missing data.
Results
Patient and overdose characteristics
A total of 545 patients fulfilled the inclusion criteria. Median age was 31 (22–43) years, and 341 (62.6%) patients were female. Median time from overdose to presentation was 3h 25min (1h 44min–6h 47min). Plasma paracetamol concentrations were available in 529 (97%) patients; in four individuals the samples had haemolysed and were not repeated, and in 12 individuals they were not performed. Median plasma paracetamol concentration was 119 mg l−1 (66–182), and time from exposure to measurement was 5h 47min (4h 36min–9h 5min). The median extrapolated 4‐h concentration was 190.0 (126.8–273.5) mg l−1.
Reported ingested dose of paracetamol
The reported ingested dose was recorded in 520 (95.4%) cases, with a median of 16 (12.5–25) g. A total of 104 patients (20.0%) took a dose ≥30 g, and the maximum ingested dose was 141 g. Reported ingested doses correlated with extrapolated 4‐h plasma concentrations (r = 0.367, P < 0.0001; Figure 1a).
Figure 1.
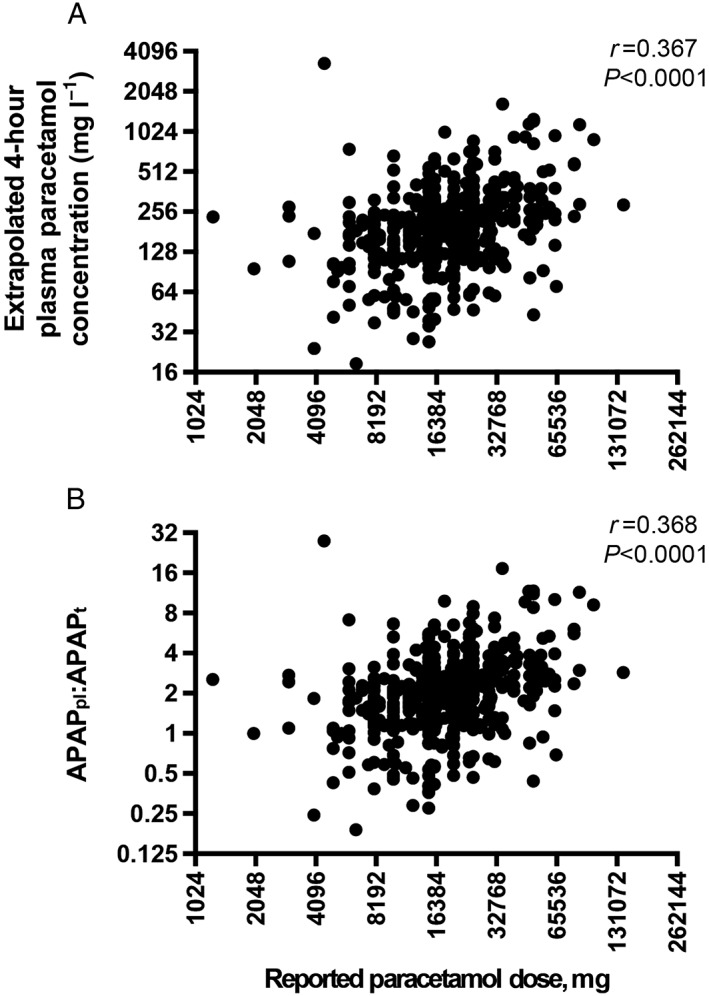
Correlations between reported dose of paracetamol ingested and (A) extrapolated 4‐h plasma paracetamol concentrations and (B) APAPpl:APAPt
APAPpl:APAPt ratios
Ratios were calculated in 527 (96.7%) patients; in the remainder this was not possible either due to lack of a measured plasma paracetamol concentration (n = 4) or due to late presentation beyond the time limits of treatment nomograms (n = 14). The median ratio was 1.94 (1.30–2.77). This measure correlated strongly with 4‐h extrapolated plasma concentrations (r = 0.999, P < 0.0001), and moderately with reported dose (r = 0.368, P < 0.0001; Figure 1b).
Prevalence of organ injury
Peak serum aminotransferase concentrations, INR and creatinine results were available in 538, 540 and 542 patients, respectively. A total of 117 (21.5%) patients had peak serum aminotransferase concentrations >2‐fold ULN; 69 (12.8%) >3‐fold ULN; and 20 (3.7%) >1000 IU l−1. Forty‐nine (9.1%) had a peak INR >1.3; and two (0.4%) >6.5. Nine (1.7%) had significant acute kidney injury with a creatinine >150 μmol l−1, and four (0.7%) >300 μmol l−1. Additional acetylcysteine beyond the standard regimen was administered to 53 (9.7%) patients. All patients recovered from the acute episode of poisoning, except for one individual who presented 13h 8min after reported ingestion of 24 g paracetamol and ethanol, and developed chronic renal impairment requiring long‐term renal replacement therapy. This patient also had acute liver failure with a serum aminotransferase concentration of 8509 IU l−1 and INR of 3.32, although hepatic function subsequently recovered and was normal at the time of hospital discharge. No patients died as a result of the acute episode of poisoning.
Relationship between estimates of overdose and development of organ injury
Patient demographics described by nomogram group (according to extrapolated 4‐h plasma paracetamol concentrations) are shown in Table 1. Correlations between reported ingested dose, 4‐h extrapolated plasma paracetamol concentrations, APAPpl:APAPt and the various outcome measures were assessed (Table 2). Sensitivities, specificities, positive predictive values and odds ratios for different APAPpl:APAPt thresholds for identifying serum aminotransferase rises >2‐fold ULN (promoting extended acetylcysteine infusion) are reported in Table 3.
Table 1.
Patient demographics, features of overdose and liver injury according to extrapolated 4‐h plasma paracetamol concentration
| 4‐hour extrapolated plasma paracetamol concentration (mg l−1) | ||||||
|---|---|---|---|---|---|---|
| <100 | 100–150 | 150–200 | 200–300 | 300–500 | >500 | |
| Cases | 68 | 111 | 101 | 137 | 73 | 39 |
| Sex (% male) | 50.0% | 40.5% | 29.7% | 39.4% | 31.5% | 30.8% |
| Median (IQR) age (years) | 30 (23–40) | 32 (22–45) | 28 (20–40) | 29 (23–43) | 31 (24–39) | 42 (25–52) |
| Median (IQR) admission paracetamol level (mg l −1 ) | 41 (24–56) | 101 (71–115) | 149 (108–168) | 183 (129–212) | 189 (93–257) | 152 (104–288) |
| Median (IQR) ingestion to paracetamol level (hours:minutes) | 5:38 (4:33–8:22) | 5:08 (4:26–7:05) | 4:49 (4:20–6:49) | 5:26 (4:38–7:32) | 7:26 (5:39–12:16) | 13:39 (9:32–18:30) |
| Median (IQR) ingestion to acetylcysteine initiation (hours:minutes) | 7:17 (5:12–9:15) | 7:45 (6:02–9:45) | 7:10 (6:00–9:19) | 7:30 (6:00–9:44) | 9:00 (7:16–14:00) | 15:32 (10:57–19:26) |
| Normal serum aminotransferases | 85.3% | 87.4% | 80.2% | 81.8% | 61.6% | 33.3% |
| Acute liver injury | 4.4% | 3.6% | 5.9% | 4.4% | 21.9% | 53.8% |
| Hepatotoxicity | 0.0% | 0.0% | 1.0% | 1.5% | 6.8% | 30.8% |
Table 2.
Correlations between reported dose, extrapolated 4‐h plasma paracetamol concentrations, APAPpl:APAPt ratio, and time to acetylcysteine with peak serum aminotransferase concentrations, INR and creatinine
| Peak serum aminotransferase concentration, IU l −1 | Peak INR | Peak serum creatinine, μmol l −1 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| r | 95% CI | P | r | 95% CI | P | r | 95% CI | P | |
| Reported dose, mg | 0.212 | 0.125−0.296 | <0.0001 | 0.034 | 0.055−0.122 | 0.44 | 0.138 | 0.050−0.224 | 0.002 |
| Extrapolated 4‐h plasma concentration, mg l −1 | 0.297 | 0.214−0.375 | <0.0001 | 0.319 | 0.237−0.396 | <0.0001 | 0.097 | 0.009−0.183 | 0.03 |
| APAP pl :APAP t | 0.286 | 0.202−0.365 | <0.0001 | 0.314 | 0.232−0.391 | <0.0001 | 0.090 | 0.002−0.177 | 0.04 |
| Time to acetylcysteine, h | 0.168 | 0.079−0.255 | 0.0002 | 0.153 | 0.064−0.241 | 0.0006 | 0.087 | −0.004−0.176 | 0.05 |
Table 3.
Performance of different APAPpl:APAPt thresholds for identification of serum aminotransferase elevations >2‐fold ULN
| APAP pl :APAP t | Sensitivity | Specificity | Positive predictive value | Odds ratio (95% CI) | P‐value |
|---|---|---|---|---|---|
| >1 | 0.95 | 0.14 | 0.13 | 3.13 (0.99–9.78) | 0.06 |
| >2 | 0.94 | 0.20 | 0.56 | 4.05 (2.28–7.22) | <0.0001 |
| >3 | 0.93 | 0.35 | 0.84 | 7.15 (4.20–12.06) | <0.0001 |
| >4 | 0.91 | 0.46 | 0.93 | 8.65 (4.77–15.55) | <0.0001 |
| >5 | 0.90 | 0.54 | 0.96 | 11.06 (2.75–21.31) | <0.0001 |
| >6 | 0.90 | 0.62 | 0.98 | 13.93 (6.24–31.79) | <0.0001 |
Relationship to reported ingested dose of paracetamol
Reported ingested dose correlated with peak serum aminotransferase concentrations (r = 0.212, P < 0.0001; Figure 2a) and creatinine (r = 0.138, P = 0.002; Figure 2b), but not INR (r = 0.034, P = ns; Figure 2c). Median peak serum aminotransferase concentration was 23 IU l−1 (16.75–39.25) in patients reporting overdoses under 30 g, and 29 IU l−1 (22–73) in those who had taken ≥30 g (P = 0.001). Reported dose did not reliably differentiate the different grades of liver injury (Figure 2d). Median INR was 1.1 in both patients taking ≥30 g paracetamol and also those reporting non‐massive overdoses (IQR 1.02–1.18 and 1.03–1.19, respectively). Median serum creatinine was 65 μmol l−1 (57–75) in patients reporting ingestions <30 g and 72.5 μmol l−1 (63.25–82.75) in those who reported ingestion of ≥30 g (P < 0.0001), but there was no difference in the frequency of creatinine rises over 150 μmol l−1 (<30 g, n = 5; ≥30 g, n = 3) or 300 μmol l−1 (<30 g, n = 1; ≥30 g, n = 2) between these groups.
Figure 2.
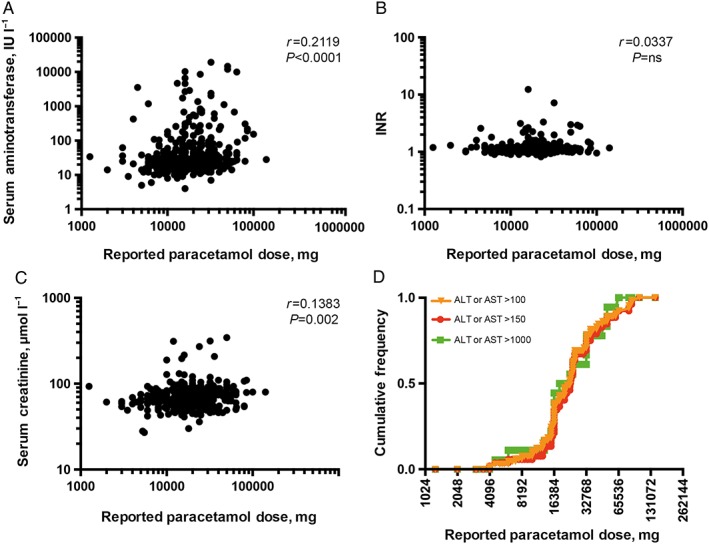
Relationship between reported dose of paracetamol ingested and (A) serum aminotransferase concentration, (B) INR and (C) serum creatinine; (D) cumulative frequency of different grades of liver injury with reported dose
Relationship to APAPpl:APAPt
APAPpl:APAPt ratio correlated with peak serum aminotransferase concentration (r = 0.286, P < 0.0001; Figure 3a), INR (r = 0.314, P < 0.0001; Figure 3b) and creatinine concentration (r = 0.090, P = 0.04; Figure 3c). There were associations between increasing APAPpl:APAPt and liver injury (Figure 4a–c), coagulopathy (Figure 4d) and acute kidney injury. A ratio of ≥3 was associated with an OR of 7.15 (4.20–12.06; P < 0.0001) for peak serum aminotransferase concentrations >2‐fold ULN; 9.19 (5.04–16.68; P < 0.0001) for acute liver injury; 35.95 (8.80–158.1; P < 0.0001) for hepatotoxicity; 8.34 (4.43–15.85; P < 0.0001) for coagulopathy; and 4.69 (1.38–15.44; P = 0.03) for acute kidney injury.
Figure 3.
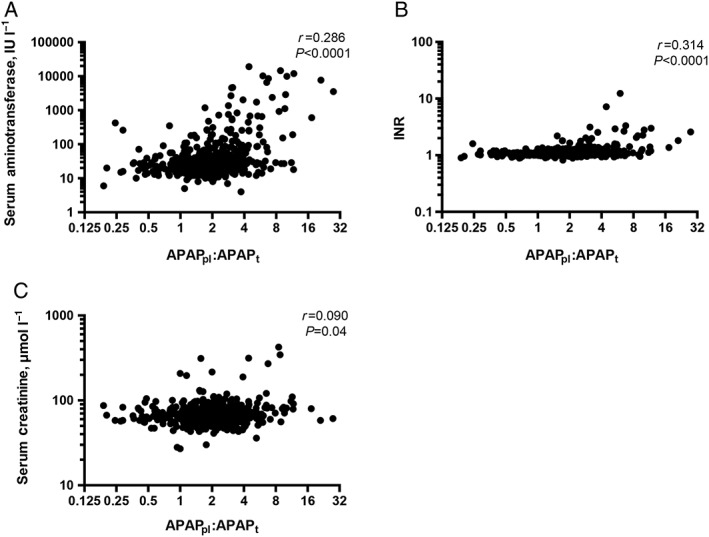
Relationship between APAPpl:APAPt and (A) serum aminotransferase concentration, (B) INR and (C) serum creatinine
Figure 4.
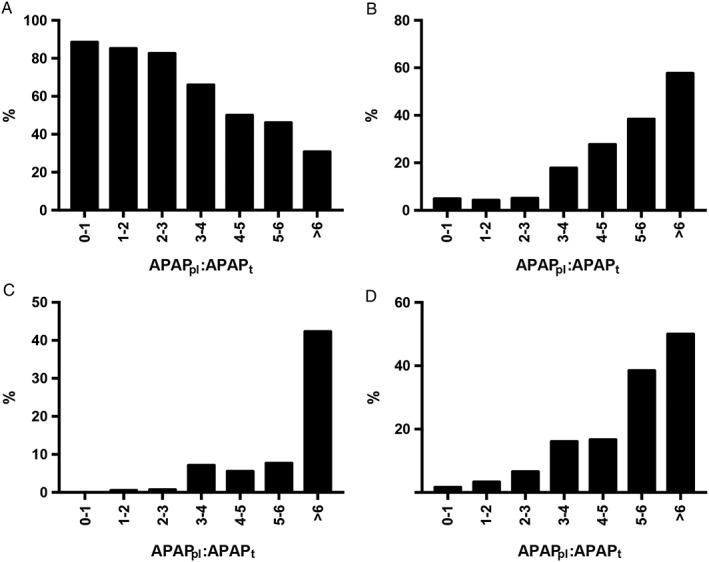
Percentage of patients in each APAPpl:APAPt group with (A) no liver injury (serum aminotransferase concentrations <50 IU l−1), (B) acute liver injury, (C) hepatotoxicity and (D) coagulopathy
Correspondingly, values for APAPpl:APAPt ratios ≥6 were 13.93 (6.24–31.79; P < 0.0001) for aminotransferase rises >2‐fold ULN; 15.94 (6.97–35.32; P < 0.0001) for acute liver injury; 44.64 (15.0–121.5; P < 0.0001) for hepatotoxicity; 13.59 (5.84–32.33; P < 0.0001) for coagulopathy; and 10.65 (2.75–39.12; P = 0.008) for acute kidney injury.
Time to acetylcysteine and outcomes
Median time to acetylcysteine was 8h 30min (6h 24min–12h 36min) in male patients and 7h 42min (6h 0min–10h 18min) in female patients (P = 0.03). Time to treatment correlated with serum aminotransferase concentration (r = 0.168 P = 0.0002), INR (r = 0.153, P = 0.0006) and serum creatinine (r = 0.087, P = 0.05).
We subsequently restricted analyses to the 248 patients who received acetylcysteine within 8 h of reported paracetamol ingestion (Table 4). The association between reported ingested dose and serum aminotransferase concentration persisted (r = 0.153, P = 0.02), as did those between APAPpl:APAPt and serum aminotransferases or INR. An APAPpl:APAPt ≥ 3 remained predictive of organ injury with an OR of 5.25 (1.98–13.13; P = 0.002) for aminotransferase rise >2‐fold ULN; 4.70 (1.66–14.48; P = 0.02) for acute liver injury; ∞ (3.56–∞; P = 0.01) for hepatotoxicity; and 5.21 (1.60–18.3; P = 0.02) for coagulopathy.
Table 4.
Correlations between reported dose and extrapolated 4‐h plasma paracetamol concentrations, APAPpl:APAPt ratio, and markers of organ injury in patients receiving acetylcysteine within 8 h of overdose
| Peak serum aminotransferase concentration, IU l −1 | Peak INR | Peak serum creatinine, μmol l −1 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| r | 95% CI | P | r | 95% CI | P | r | 95% CI | P | |
| Reported dose, mg | 0.153 | 0.025−0.276 | 0.02 | −0.040 | −0.167−0.089 | 0.53 | 0.108 | −0.021−0.233 | 0.09 |
| Extrapolated 4‐h plasma concentration, mg l −1 | 0.184 | 0.059−0.304 | 0.003 | 0.271 | 0.150−0.385 | <0.0001 | −0.029 | −0.155−0.098 | 0.65 |
| APAP pl :APAP t | 0.185 | 0.060−0.305 | 0.003 | 0.269 | 0.232−0.391 | <0.0001 | −0.027 | −0.153−0.100 | 0.67 |
By comparison, in patients who received acetylcysteine later than 8 h from reported ingestion, APAPpl:APAPt ≥ 3 had an OR of 8.61 (3.90–18.23; P < 0.0001) for aminotransferase rises >2‐fold ULN; 11.38 (4.91–25.36; P < 0.0001) for acute liver injury; 18.88 (4.73–84.67; P < 0.0001) for hepatotoxicity; and 9.46 (4.00–21.29; P < 0.0001) for coagulopathy.
Discussion
Although the current regimen of acetylcysteine for treating paracetamol overdose has been extremely successful, the continued use of a standard protocol for every case has been questioned 5, 8, 9. In particular, it has been suggested that patients who have taken very large overdoses may require higher doses of acetylcysteine, or protracted infusions. Intravenous doses up to 980 mg kg−1 acetylcysteine over 48 h have previously been used safely 25, notwithstanding evidence from one animal model that suggested prolonged therapy might delay recovery from hepatotoxicity 26. It is known that NAPQI generation rises with increasing paracetamol dose, and also that hepatic injury prolongs paracetamol half‐life. Furthermore, there are several case reports, and one observational study, of patients developing hepatotoxicity despite receiving acetylcysteine within 8 h of reported overdose 12, 13, 14, 15, 16.
Our study systematically assessed outcomes of massive paracetamol overdose. Key findings were that, despite receiving standard therapy with acetylcysteine, patients with massive overdoses were more likely to develop significant liver and kidney injury, and coagulopathy. APAPpl:APAPt ratio was a better predictor of organ toxicity than the reported dose ingested. Although overall correlations with outcomes were modest in magnitude, and differences in medians (while statistically significant) were of limited clinical relevance, this did provide a tool for distinguishing higher and lower risk groups. This persisted even when acetylcysteine was administered within 8 h of reported ingestion, demonstrating that while time to treatment was a strong predictor of organ injury, it was not the sole determining factor in early presenting poisoning. These findings validate and extend, in an independent cohort, those recently published by a specialist toxicology unit in Edinburgh 16. The case features in our patients were broadly similar, except that liver injury and hepatotoxicity were more frequent in the highest concentration subgroups in our study; this may relate to the higher measured paracetamol concentrations at the times of presentation.
The original acetylcysteine treatment regimen was constructed based on empirical considerations 4, 7. Although effective for the majority of patients, it is not clear that the implicit assumptions necessarily hold true in massive overdose. In such patients, absorption of paracetamol may be delayed: this could be due to direct effects of paracetamol on gastric motility 27; co‐ingestion of other drugs such as opiates or anticholinergics that delay gastric emptying 28; insufficient volume of gastric secretions to solubilize large quantities of paracetamol 29; or formation of a pharmacological bezoar 13. The half‐life of paracetamol can progressively extend as hepatotoxicity develops, such that significant quantities of NAPQI could be generated after the 16‐h acetylcysteine infusion has finished 30. Finally, there is evidence from animal models that paracetamol may undergo enterohepatic recirculation, with hydrolysis of non‐toxic conjugates by gut flora and reabsorption of the parent drug 31. These factors likely explain, alone or in combination, the double peaks of plasma paracetamol reported following large overdoses 13. In some of these patients, the second peak can occur in excess of 30 h after ingestion, and these individuals are more likely to develop hepatotoxicity despite early acetylcysteine therapy.
While there has been considerable recent interest in the development of novel early biomarkers, such as miRNA‐122, to further stratify those at high risk of tissue injury and guide management, there is a possibility these might fail to identify cases if a major contributor to adverse outcomes in massive paracetamol overdose is a delay in the pharmacokinetic profile 32. This is also relevant when considering adoption of an abbreviated acetylcysteine protocol 33, and might mandate protracted observation in people who have taken massive doses. It is important that this patient cohort is specifically considered when evaluating proposed changes to practice.
There are a few limitations to the current study. Principal among these is the reliance on an accurate patient history and medical documentation at the time of clinical review, particularly as regards paracetamol dose and time of ingestion. As the database is clinical, there is a risk of misclassification as data are not validated at the time of entry, although one strength of this approach is that data entry is blinded to the study question. The correlations between reported doses, extrapolated 4‐h plasma paracetamol concentrations and APAPpl:APAPt provide some reassurance that these possess a reasonable degree of reliability, although concordance was lower than in previously reported series 34 and there were a number of outliers. These could result from errors in patient estimation of dose or calculation by the admitting physician, or by an increase in the half‐life of paracetamol as has been documented previously in patients with significant paracetamol toxicity, thus introducing inaccuracies into extrapolation of paracetamol concentrations.
Secondly, blood tests for paracetamol, liver, coagulation and renal function were performed routinely during clinical practice at presentation to the Emergency Department, as well as on completion of the standard acetylcysteine regimen, and were thus not completely systematic. In the absence of more frequent testing, it is possible that in some cases peak values may have been missed. In addition, at our hospital at the time of this study, paracetamol concentrations were not repeated during or after treatment, so it is not possible to comment on alterations in plasma half‐life. It was also not possible to formally grade kidney injury using RIFLE/AKIN criteria, due to the lack of baseline blood tests and limited longitudinal follow‐up in this patient cohort.
Third, prior to 2012, the acetylcysteine protocol required calculations to be performed by both the prescribing physician, as well as the administering nurses. This process is error prone 35, and hence it is possible that some patients nominally receiving early treatment were in fact under‐dosed. Finally, the assumptions underlying extrapolation of 4‐h paracetamol concentrations may break down if paracetamol metabolism changes in a nonlinear fashion or becomes saturated at very high doses, or should a double peak phenomenon exist widely.
These findings are clinically important, as they suggest that under current protocols patients taking massive paracetamol overdoses may be undertreated, and that either an increase in the dose intensity and/or duration of acetylcysteine therapy could be beneficial. A high APAPpl:APAPt ratio is associated with increased risk and therefore further consideration should be given to alternative acetylcysteine treatment strategies in these patients. Risks of organ injury rose with an APAPpl:APAPt (based on a treatment line through 100 mg l−1 at 4 h) ≥3, and a ratio ≥ 6 was strongly predictive. Based on analysis of the sensitivities and positive predictive values of different threshold ratios, we believe on balance that the former cutoff should be used to define a higher risk group. The optimum strategy is not clear at present, and would require a more detailed understanding of the mechanisms responsible for the excess in organ injury despite early acetylcysteine. This could be informed by performing serial plasma paracetamol measurements in at‐risk individuals to determine whether this relates to delayed absorption, second peaks or prolonged half‐life. In the event of a significant contribution from the former, or substantial enterohepatic recirculation of the parent drug, there may also be a role for multiple doses of activated charcoal to augment gastrointestinal elimination. Novel biomarkers of liver injury and abbreviated treatment protocols should also be specifically validated in this patient cohort.
Competing Interests
All authors have completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare that P.I.D. is a member of the MHRA CHM 2016 Paracetamol Expert Working Group, and D.J.B.M. is a consultant for GSK. There are no other relationships or activities that could appear to have influenced the submitted work.
The authors would like to thank Melvin Lipi for assistance with database searches.
Contributors
P.I.D., D.M.W. and S.L.G. conceived the study; D.J.B.M., C.L.D. and A.M.D. collected data; and D.J.B.M. performed statistical analyses. All authors were involved in data interpretation, drafting and critical revision of the manuscript, and have approved the final version submitted for publication.
Marks, D. J. B. , Dargan, P. I. , Archer, J. R. H. , Davies, C. L. , Dines, A. M. , Wood, D. M. , and Greene, S. L. (2017) Outcomes from massive paracetamol overdose: a retrospective observational study. Br J Clin Pharmacol, 83: 1263–1272. doi: 10.1111/bcp.13214.
References
- 1. Southan C, Sharman JL, Benson HE, Faccenda E, Pawson AJ, Alexander SP, et al The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. Nucl Acids Res 2016; 44: D1054–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Larson AM, Polson J, Fontana RJ, Davern TJ, Lalani E, Hynan LS, et al. Acetaminophen‐induced acute liver failure: results of a United States multicenter, prospective study. Hepatology 2005; 42: 1364–1372. [DOI] [PubMed] [Google Scholar]
- 3. Lancaster EM, Hiatt JR, Zarrinpar A. Acetaminophen hepatotoxicity: an updated review. Arch Toxicol 2015; 89: 193–199. [DOI] [PubMed] [Google Scholar]
- 4. Prescott LF, Illingworth RN, Critchley JA, Stewart MJ, Adam RD, Proudfoot AT. Intravenous N‐acetylcystine: the treatment of choice for paracetamol poisoning. Br Med J 1979; 2: 1097–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chiew AL, Isbister GK, Duffull SB, Buckley NA. Evidence for the changing regimens of acetylcysteine. Br J Clin Pharmacol 2016; 81: 471–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Prescott LF. Kinetics and metabolism of paracetamol and phenacetin. Br J Clin Pharmacol 1980; 10 (Suppl 2): 291S–298S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mitchell JR, Thorgeirsson SS, Potter WZ, Jollow DJ, Keiser H. Acetaminophen‐induced hepatic injury: protective role of glutathione in man and rationale for therapy. Clin Pharmacol Ther 1974; 16: 676–684. [DOI] [PubMed] [Google Scholar]
- 8. Buckley NA, Dawson AH, Isbister GK. Treatments for paracetamol poisoning. BMJ 2016; 353: i2579. [DOI] [PubMed] [Google Scholar]
- 9. Bateman DN, Dear JW, Thomas SH. New regimens for intravenous acetylcysteine, where are we now? Clin Toxicol (Phila) 2016; 54: 75–78. [DOI] [PubMed] [Google Scholar]
- 10. Rumack BH, Bateman DN. Acetaminophen and acetylcysteine dose and duration: past, present and future. Clin Toxicol (Phila) 2012; 50: 91–98. [DOI] [PubMed] [Google Scholar]
- 11. Dart RC, Rumack BH. Patient‐tailored acetylcysteine administration. Ann Emerg Med 2007; 50: 280–281. [DOI] [PubMed] [Google Scholar]
- 12. Doyon S, Klein‐Schwartz W. Hepatotoxicity despite early administration of intravenous N‐acetylcysteine for acute acetaminophen overdose. Acad Emerg Med 2009; 16: 34–39. [DOI] [PubMed] [Google Scholar]
- 13. Hendrickson RG, McKeown NJ, West PL, Burke CR. Bactrian (‘double hump’) acetaminophen pharmacokinetics: a case series and review of the literature. J Med Toxicol 2010; 6: 337–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang GS, Monte A, Bagdure D, Heard K. Hepatic failure despite early acetylcysteine following large acetaminophen‐diphenhydramine overdose. Pediatrics 2011; 127: e1077–e1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Smilkstein MJ, Knapp GL, Kulig KW, Rumack BH. Efficacy of oral N‐acetylcysteine in the treatment of acetaminophen overdose. Analysis of the national multicenter study (1976 to 1985). N Engl J Med 1988; 319: 1557–1562. [DOI] [PubMed] [Google Scholar]
- 16. Cairney DG, Beckwith HK, Al‐Hourani K, Eddleston M, Bateman DN, Dear JW. Plasma paracetamol concentration at hospital presentation has a dose‐dependent relationship with liver injury despite prompt treatment with intravenous acetylcysteine. Clin Toxicol (Phila) 2016; 54: 405–410. [DOI] [PubMed] [Google Scholar]
- 17. Greene SL, Wood DM, Gawarammana IB, Warren‐Gash C, Drake N, Jones AL, et al. Improvement in the management of acutely poisoned patients using an electronic database, prospective audit and targeted educational intervention. Postgrad Med J 2008; 84: 603–608. [DOI] [PubMed] [Google Scholar]
- 18. Waring WS, Stephen AF, Robinson OD, Dow MA, Pettie JM. Serum urea concentration and the risk of hepatotoxicity after paracetamol overdose. QJM 2008; 101: 359–363. [DOI] [PubMed] [Google Scholar]
- 19. Bateman DN, Carroll R, Pettie J, Yamamoto T, Elamin ME, Peart L, et al. Effect of the UK's revised paracetamol poisoning management guidelines on admissions, adverse reactions and costs of treatment. Br J Clin Pharmacol 2014; 78: 610–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bateman DN. Paracetamol poisoning: beyond the nomogram. Br J Clin Pharmacol 2015; 80: 45–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Robles‐Diaz M, Lucena MI, Kaplowitz N, Stephens C, Medina‐Caliz I, Gonzalez‐Jimenez A, et al. Use of Hy's law and a new composite algorithm to predict acute liver failure in patients with drug‐induced liver injury. Gastroenterology 2014; 147: 109–18.e5. [DOI] [PubMed] [Google Scholar]
- 22. Yarema MC, Johnson DW, Berlin RJ, Sivilotti ML, Nettel‐Aguirre A, Brant RF, et al. Comparison of the 20‐hour intravenous and 72‐hour oral acetylcysteine protocols for the treatment of acute acetaminophen poisoning. Ann Emerg Med 2009; 54: 606–614. [DOI] [PubMed] [Google Scholar]
- 23. Ferner RE, Dear JW, Bateman DN. Management of paracetamol poisoning. BMJ 2011; 342: d2218. [DOI] [PubMed] [Google Scholar]
- 24. Ali T, Tachibana A, Khan I, Townend J, Prescott GJ, Smith WC, et al. The changing pattern of referral in acute kidney injury. QJM 2011; 104: 497–503. [DOI] [PubMed] [Google Scholar]
- 25. Heard K, Rumack BH, Green JL, Bucher‐Bartelson B, Heard S, Bronstein AC, et al. A single‐arm clinical trial of a 48‐hour intravenous N‐acetylcysteine protocol for treatment of acetaminophen poisoning. Clin Toxicol (Phila) 2014; 52: 512–518. [DOI] [PubMed] [Google Scholar]
- 26. Yang R, Miki K, He X, Killeen ME, Fink MP. Prolonged treatment with N‐acetylcystine delays liver recovery from acetaminophen hepatotoxicity. Crit Care 2009; 13: R55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Adams BK, Mann MD, Aboo A, Isaacs S, Evans A. Prolonged gastric emptying half‐time and gastric hypomotility after drug overdose. Am J Emerg Med 2004; 22: 548–554. [DOI] [PubMed] [Google Scholar]
- 28. Kirschner RI, Rozier CM, Smith LM, Jacobitz KL. Nomogram line crossing after acetaminophen combination product overdose. Clin Toxicol (Phila) 2016; 54: 40–46. [DOI] [PubMed] [Google Scholar]
- 29. Smith SW, Howland MA, Hoffman RS, Nelson LS. Acetaminophen overdose with altered acetaminophen pharmacokinetics and hepatotoxicity associated with premature cessation of intravenous N‐acetylcysteine therapy. Ann Pharmacother 2008; 42: 1333–1339. [DOI] [PubMed] [Google Scholar]
- 30. Schiodt FV, Ott P, Christensen E, Bondesen S. The value of plasma acetaminophen half‐life in antidote‐treated acetaminophen overdosage. Clin Pharmacol Ther 2002; 71: 221–225. [DOI] [PubMed] [Google Scholar]
- 31. Watari N, Iwai M, Kaneniwa N. Pharmacokinetic study of the fate of acetaminophen and its conjugates in rats. J Pharmacokinet Biopharm 1983; 11: 245–272. [DOI] [PubMed] [Google Scholar]
- 32. Dear JW, Antoine DJ, Starkey‐Lewis P, Goldring CE, Park BK. Early detection of paracetamol toxicity using circulating liver microRNA and markers of cell necrosis. Br J Clin Pharmacol 2014; 77: 904–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bateman DN, Dear JW, Thanacoody HK, Thomas SH, Eddleston M, Sandilands EA, et al. Reduction of adverse effects from intravenous acetylcysteine treatment for paracetamol poisoning: a randomised controlled trial. Lancet 2014; 383: 697–704. [DOI] [PubMed] [Google Scholar]
- 34. Waring WS, Robinson OD, Stephen AF, Dow MA, Pettie JM. Does the patient history predict hepatotoxicity after acute paracetamol overdose? QJM 2008; 101: 121–125. [DOI] [PubMed] [Google Scholar]
- 35. Selvan VA, Calvert SH, Cavell G, Glucksman E, Kerins M, Gonzalez J. Weight‐based N‐acetylcysteine dosing chart to minimise the risk of calculation errors in prescribing and preparing N‐acetylcysteine infusions for adults presenting with paracetamol overdose in the emergency department. Emerg Med J 2007; 24: 482–484. [DOI] [PMC free article] [PubMed] [Google Scholar]


