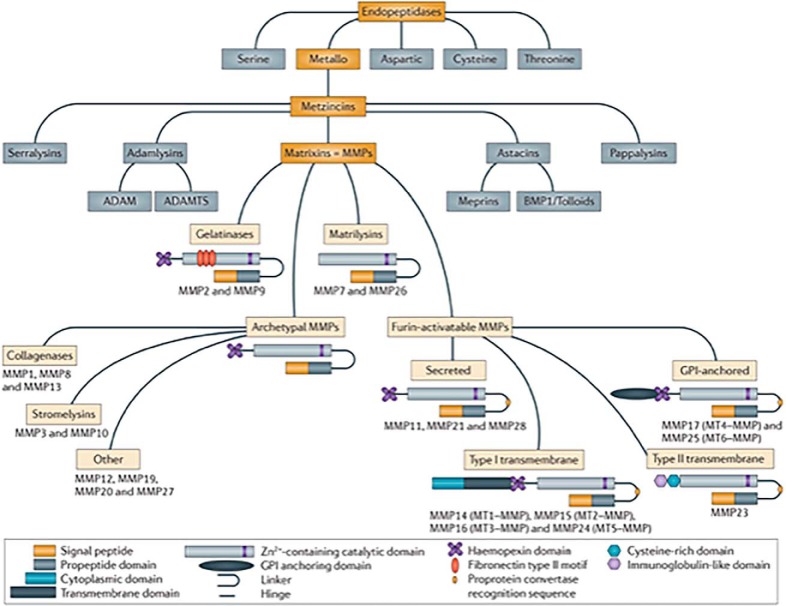
Figure 5.
MMPs, also named Matrixins, are a family within the clan of metallo-endopeptidases called the Metzincins (18, 23, 33). Other Metzincin families that largely function extracellularly include the ADAMs, the ADAM-TS, and the Astacins, including the meprins and bone morphogenetic protein 1 (BMP1). The MMPs share a common domain structure: the pre-domain that contains a signal peptide responsible for secretion; the pro-domain that keeps the enzyme inactive by an interaction between a cysteine residue and the Zn2+ ion group from the catalytic domain; and the hemopexin-like C-terminal domain, which is linked to the catalytic domain by a flexible hinge region. MMP7 and MMP26 lack the hinge region and the hemopexin domain. MMP2 and MMP9 contain a fibronectin type II motif inserted into the catalytic site, and MT-MMPs have a transmembrane domain or a glycosylphosphatidylinositol (GPI) anchor at the C terminus. MMP23 has unique features: the N-terminal signal anchor that targets MMP23 to the cell membrane, a cysteine array, and an immunoglobulin-like domain. Figure from Vandenbrouke and Libert (30). Reprinted by permission from Macmillan Publishers Ltd.: Nature Drug Discovery 13, 904–927 copyright (2014).