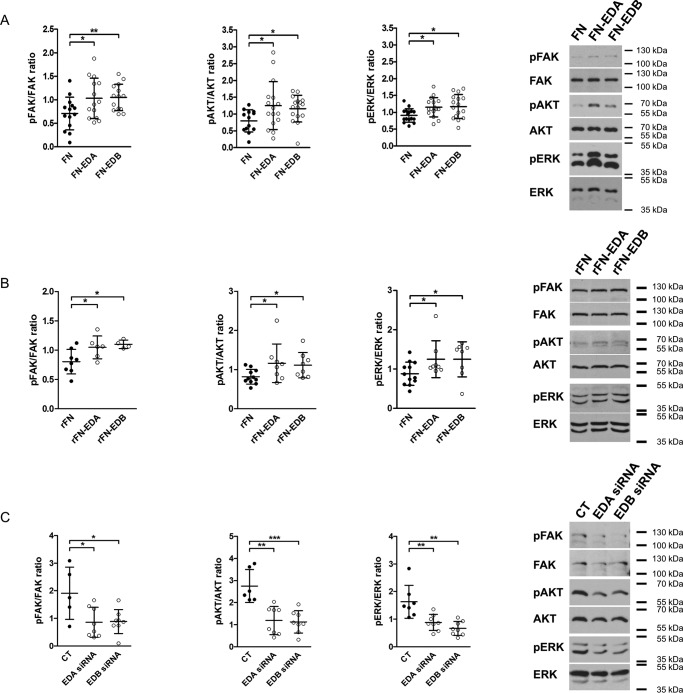
Figure 4.
EDA- or EDB-containing fibronectin increase integrin signaling, whereas silencing them diminishes signaling. A, expression of FN-EDA or FN-EDB in osteoblasts enhances FAK, AKT, and ERK phosphorylation compared with cells transfected with the control construct lacking the EDA and the EDB domains (FN) (biological replicates for FAK: n = 14/14/14 in 3 experiments; AKT: n = 13/16/16 in 6 experiments; and ERK: n = 16/16/16 in 5 experiments). B, exposing osteoblasts to rFN-EDA or rFN-EDB similarly enhances FAK, AKT, and ERK phosphorylation compared with the control construct (rFN) lacking both EDA and EDB domains (biological replicates for FAK: n = 8/6/4; AKT: n = 11/8/8; and ERK: n = 12/8/8 in 4 experiments). Cells were exposed to 200 ng/ml recombinant proteins produced as outlined under “Experimental procedures” for 15 min after culture in serum-free medium for 8 h. C, silencing EDA- or EDB-containing fibronectin using siRNA diminished integrin-mediated signaling (biological replicates for FAK: n = 5/8/8; AKT: n = 6/8/8; and ERK: n = 7/8/8 each in 2 experiments). Osteoblasts were transfected and cultured for 2 days in medium containing fibronectin-depleted FCS, and cell lysates were collected in A and C. Results are expressed as mean ± S.D. (error bars). *, p < 0.05; **, p < 0.01; ***, p < 0.005.