Figure 8.
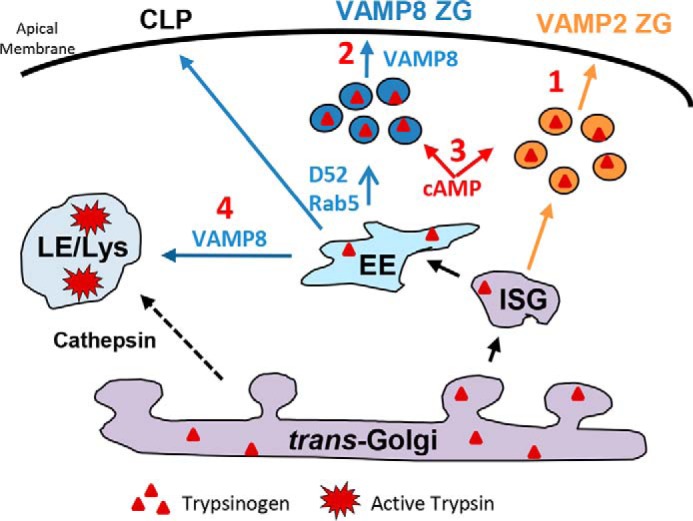
Loss of VAMP8-dependent secretion leads to intracellular trypsinogen activation. Immature secretory granules (ISG) give rise to VAMP2- and VAMP8-contianing ZGs. Under normal conditions acinar stimulation is characterized by VAMP2-mediated ZG exocytosis at <2 min (1) and VAMP8-dependent ZG exocytosis at later times (2). Elevated cAMP augments early and late phases of maximally stimulated secretion. With supramaximal stimulation, VAMP8-mediated late-phase secretion is compromised, and this inhibition is alleviated by elevated cAMP (3). The early endosomal proteins, Rab5 and D52, are necessary for VAMP8-dependent secretion and also promote basal secretion from an endolysosome-like secretory compartment termed the constitutive-like pathway (CLP) (1, 21). Inhibition of VAMP8-dependent secretion leads to an accumulation of intracellular trypsinogen that traffics from early (EE) to the late endosome (LE)/lysosome (Lys), also involving VAMP8-dependent fusion events. Cathepsin B is directly trafficked from the Golgi to the LE/lysosome by a mannose 6-phosphate receptor pathway where it mixes with trypsinogen to produce active trypsin leading to the pathogenesis of pancreatitis.
