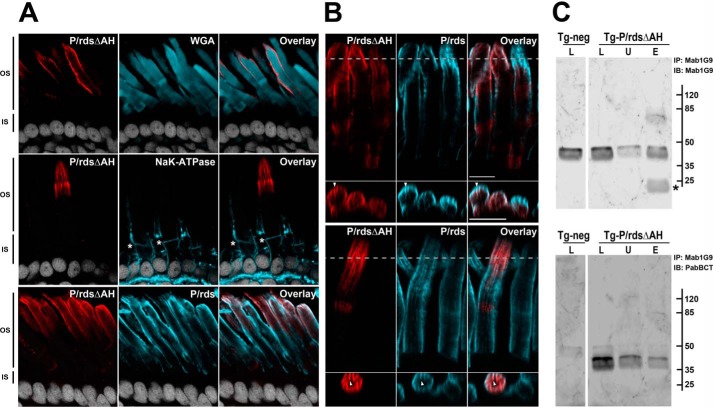
Figure 4.
P/rdsΔAH is efficiently trafficked to rod photoreceptor OS disk rims in transgenic X. laevis. A, confocal images (single optical sections) of transgenic X. laevis photoreceptors analyzed by IHC. Ocular cryosections were double-labeled for the P/rdsΔAH mutant (red) and the OS marker protein WGA (cyan, upper panels), or the P/rdsΔAH mutant (red) and the inner segment marker protein Na+,K+-ATPase (cyan, middle panels), or the P/rdsΔAH mutant (red) and endogenous WT P/rds (cyan, lower panels). In each case, nuclei were counterstained with Hoechst 33342 (white). OS and inner segment boundaries are indicated with asterisks (*, middle panels). In every case, P/rdsΔAH localization was restricted to OSs. B, detailed views of IHC double labeling of P/rdsΔAH mutant (red) and endogenous WT P/rds (cyan) in transgenic photoreceptors; upper and lower panels show representative data from different cells expressing the transgenic protein. Longitudinal views (above) show each protein present at OS disk edges and incisures. Cross-sectional views (below) derived from reconstructed volumes (taken at dotted lines) show overlapping distribution patterns in incisures (arrowheads). C, Mab1G9 immunoprecipitation of endogenous frog P/rds from retinal lysates derived from transgenic (Tg-P/rdsΔAH) and non-transgenic (Tg-neg) X. laevis. The lysed (L), unbound (U), and eluted (E) fractions were assayed to measure the efficiency of the pulldown reaction for the endogenous WT P/rds (Mab1G9; upper panel) and to determine whether the transgenic P/rdsΔAH co-precipitated (PabBCT; lower panel). Although nearly all endogenous WT P/rds was immunoprecipitated (IP) (upper panel), the great majority of the mutant remained in the unbound fraction (lower panel) and was therefore trafficked to the OS in an independent manner. Asterisk (*; upper panel) indicates antibody light chain eluted from the Mab1G9-Sepharose beads used for immunoprecipitation. IB, immunoblot.