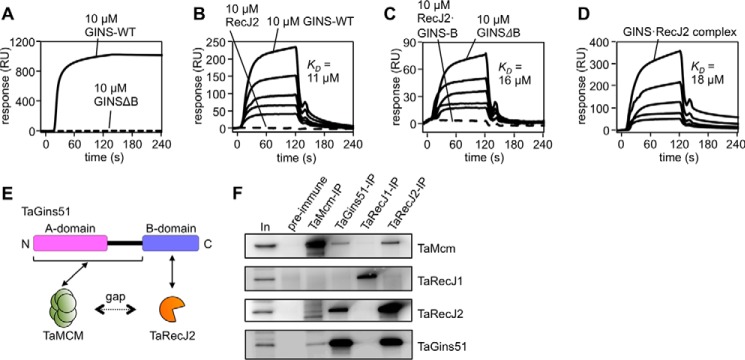
Figure 5.
Physical interactions of TaRecJ2 with TaGINS and TaMCM. A--D, SPR analyses were performed. Purified TaRecJ2 (A) and TaMCM (B--D) were each immobilized on CM5 Sensor Chips. The loaded proteins are shown at the top of the sensorgrams. A, TaGINS-WT and TaGINSΔB (10 μm each) were loaded onto the chip for 120 s. RU, resonance units. B, various concentrations (10, 5, 2.5, 1.25, and 0.63 μm) of TaGINS-WT and TaRecJ2 (10 μm) were loaded onto the chip for 120 s. C, Various concentrations (10, 5, 2.5, 1.25, and 0.63 μm) of TaGINSΔB and the TaRecJ2·TaGINS-B-domain complex (10 μm) were loaded onto the chip for 120 s. D, various concentrations (10, 5, 2.5, 1.25, and 0.63 μm as a 2:1 complex) of the TaRecJ2·TaGINS complex were loaded onto the chip for 120 s. The calculated KD values from these SPR analyses are shown in each panel. E, the scheme for interactions among TaGins51, TaMCM, and TaRecJ2. Formation of the CMG-like complex depends on bridging the gap between TaMCM and TaRecJ2 by TaGins51. F, Immunoprecipitation analyses were performed to confirm the formation of a complex including TaMCM, TaGINS, TaRecJ1, and TaRecJ2 in the T. acidophilum cell extract. The immunocomplexes were captured with anti-TaMcm, anti-TaGins51, anti-TaRecJ1, and anti-TaRecJ2 antibodies, respectively, from the whole cell extract (as shown on the top) and were subjected to 10–20% SDS-PAGE followed by Western blot analyses using these antibodies (shown on the right side). The whole cell extracts without immunoprecipitation (In) or precipitated with DynaBeads Protein G (Novex) treated with pre-immune serum were also loaded as positive and negative controls, respectively.