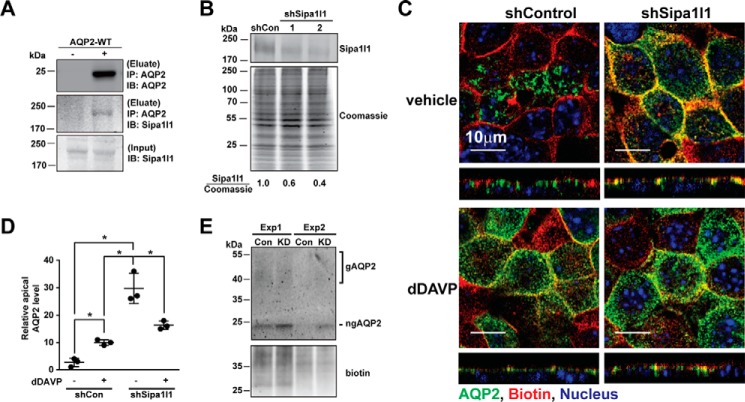
Figure 5.
Sipa1l1 participated in AQP2 endocytosis. A, immunoprecipitation (IP) followed by immunoblotting (IB) for testing AQP2-Sipa1l1 interaction. The mpkCCD cells were transfected with or without the wild-type AQP2 expression vector before the cell lysates were subjected to immunoprecipitation with the AQP2 antibody followed by immunoblotting for AQP2 and Sipa1l1. B, representative immunoblotting for Sipa1l1 in non-targeted control (shCon, TRCN0000072246: CAAATCACAGAATCGTCGTAT) and Sipa1l1 knockdown (shSipa1l1) mpkCCD cells. Two shSipa1l1 sequences (TRCN0000106106, GCCATTATGAACAGACATAAT; and TRCN0000106109, CGCCACTAGCAAATATCTGAT) were used for the knockdown. The Sipa1l1 band intensity was measured with the LiCor Odyssey scanner and normalized with Coomassie staining. Sipa1l1 knowndown efficiency is indicated. Three independent experiments were performed. C, confocal immunofluorescence staining for AQP2 in control and Sipa1l1 knockdown mpkCCD cells in response to vehicle or 1 nm dDAVP for 1 h. D, a scatter plot of the imaging results. The analysis method was described above in Fig. 2C. Values are mean ± S.D. Asterisk indicates significance p < 0.05, t test. E, immunoblotting for apical AQP2. The mpkCCD cells, control (Con) or Sipa1l1 knockdown (KD), under the vehicle control conditions were labeled with apical surface biotinylation before the labeled protein was purified with streptavidin affinity chromatography for immunoblotting and biotin staining. Two experiments (Exp 1 and 2) were done. gAQP2, glycosylated AQP2; ngAQP2, non-glycosylated AQP2.