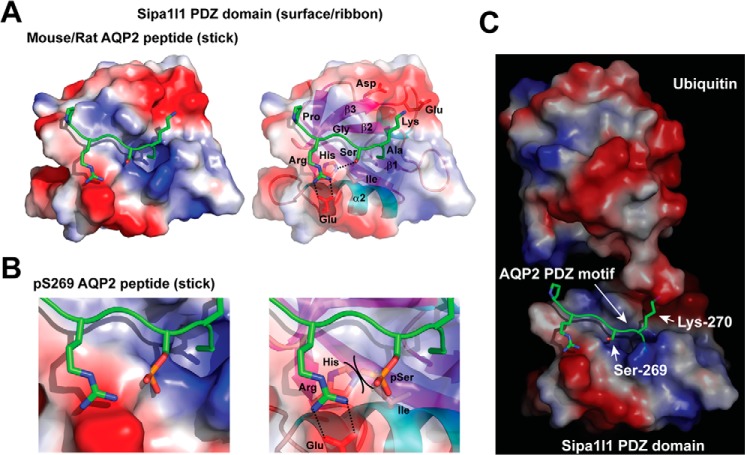
Figure 7.
Structural models of the Sipa1l1 PDZ domain. A, Sipa1l1 PDZ domain interacting with non-phosphorylated AQP2 peptide. The Sipa1l1 PDZ domain is consisted of β1, β2, β3, α1, β4, β5, α2, and β6 secondary structures shown as a space-filled surface (left panel) and a ribbon schematic model (right panel). Positive, electroneutral, and negative surfaces are colored blue, white, and red, respectively. The murine AQP2 motif -PRGSKA is shown as sticks. Nitrogen, oxygen, and phosphorus are colored blue, red, and orange, respectively. B, a blowup model of the Sipa1l1 PDZ domain colliding the Ser-269-phosphorylated AQP2 peptide. Steric hindrance between the phosphorylated Ser (pSer) and the His side chain is indicated (right panel). C, a model of ubiquitylation at Lys-270 of the AQP2 COOH-terminal peptide bound by the Sipa1l1 PDZ domain.