Figure 3.
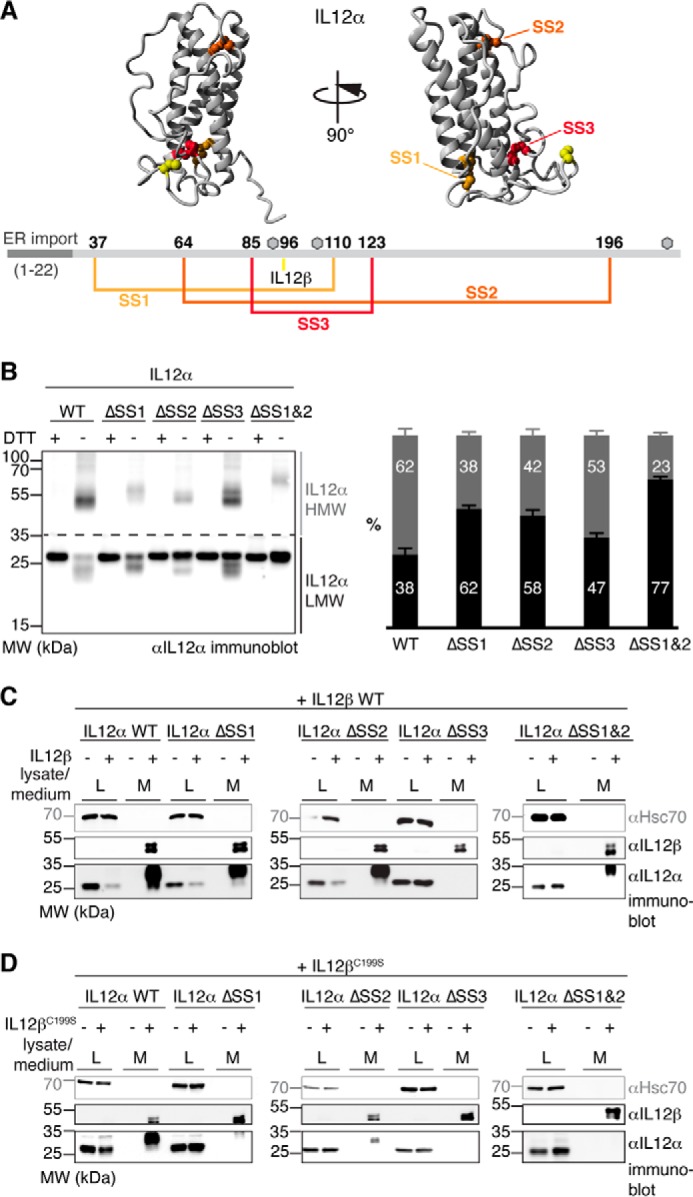
Disulfide bridges affect IL-12α misfolding and secretion differently. A, disulfide bonds within IL-12α. Cysteines forming intra- or intermolecular disulfide bridges are highlighted in IL-12α (structure based on PDB code 3HMX), numbered, and depicted in the corresponding colors in IL-12α. Gray hexagons indicate predicted glycosylation sites. B, impact of intramolecular disulfide bridges on the IL-12α redox status. IL-12α subunits individually lacking one disulfide bridge (ΔSS1–3, respectively; cysteine pairs were mutated to Ser) or a combination of two mutants (ΔSS1&2) were analyzed by non-reducing SDS-PAGE (left panel). 293T cells were transfected with the different IL-12α subunits and treated with DTT where indicated, and 2% lysate (L) was applied to the gel and blotted with IL-12α antibody. A quantitative analysis (right panel) indicates the percentage of HMW and LMW IL-12α species (n = 4 ± S.E.). LMW species (black) were defined as smaller than 35 kDa and HMW species (gray) as larger than 35 kDa. MW, molecular weight. C, secretion behavior of IL-12α disulfide bridge mutants. 293T cells were transfected with the indicated constructs, and 2% lysate or medium (M) was applied to the gel and blotted with the indicated antibodies. Hsc70 served as a loading control. D, secretion behavior of IL-12α disulfide bridge mutants. The same as in C, except secretion of IL-12α constructs in the presence of IL-12βC199S was analyzed.
