Summary
This study will test the efficacy of motivational interviewing (MI) conducted by primary care providers and dieticians among children ages 2-8 years old with a body mass index (BMI) ≥85th and ≤97th percentile. Forty-three practices from the American Academy of Pediatrics, Pediatric Research in Office Settings Network were assigned to one of three groups. Group 1 (usual care) measures BMI percentile at baseline, and at 1- and 2-year follow-ups and received standard health education materials. Group 2 providers deliver three proactive MI counselling sessions with a parent of the index child in Year 1 and one additional ‘booster’ visit in Year 2. Group 3 adds six MI counselling sessions from a trained dietician. The primary outcome is the child’s BMI percentile at 2-year follow-up. Secondary outcomes include parent report of the child’s screen time, physical activity, intake of fruits and vegetables, and sugar-sweetened beverages. We enrolled 584 eligible children whose mean BMI percentile was 92.0 and mean age of 5.1. The cohort was 57% female. Almost 70% of parents reported a household income of ≥$40 000 per year, and 39% had at least a college education. The cohort was 63% White, 23% Hispanic, 7% Black and 7% Asian. Parent self-reported confidence that their child will achieve a healthy weight was on average an 8 (out of 10). To date, several aspects of the study can inform similar efforts including our ability to use volunteer clinicians to recruit participants and their willingness to dedicate their time, without pay, to receive training in MI.
Keywords: Counselling, dietician, motivational interviewing, obesity, paediatric
Introduction
Obesity and its medical and economic sequelae have risen dramatically among America’s youth over the past 30 years, although there is some indication that rates have recently plateaued (1–6). Ameliorating childhood obesity rates in this country will require concerted intervention at multiple levels, e.g. policy, schools, industry and healthcare settings (2). The paediatric primary care office represents one potentially important, although largely underutilized, delivery channel for obesity prevention and treatment services (7,8).
Paediatricians generally believe that they should be involved in the detection, prevention and treatment of childhood obesity (9), yet paediatric obesity in primary care remains under-treated (7–9). Although lack of time and reimbursement are cited as barriers to delivering treatment (9–11), these do not appear to be the most critical factors discouraging practitioners from intervening. Rather, even more pivotal appears to be their perceived lack of motivational and behavioural skills and the confidence to employ them (9–11). They also perceive considerable resistance to change on the part of children and parents, which in part leads to a perception that available treatments are ineffective (9–11). In one study (10), e.g. only 30% of MDs felt their efficacy for obesity counselling was good to excellent and only 10% felt obesity counselling was effective (10). In another study (12), only 26% of paediatricians felt ‘quite’ to ‘extremely’ competent to treat overweight youth and only 37% felt ‘quite’ to ‘extremely’ comfortable providing such treatment (12). Almost 80% of paediatricians report feeling ‘very frustrated’ treating paediatric obesity (12). One source of frustration is the lack of an on-site dietician to provide nutritional counselling (11).
Many family-based paediatric weight control interventions have been tested (13–19) but most have been conducted by psychologists, health coaches, nurses or dieticians, and most have been implemented outside of the primary care setting. Interventions have been delivered through primary care paediatric practices; however, few studies have been conducted where the primary care physician actually delivered the core intervention (15,20–25). To date, results of most major outcome studies in primary care settings have not shown significant effects on BMI (26), although effects on physical activity, sedentary and dietary behaviours have been reported (22,23). Reasons for the null effects on adiposity in prior office-based studies may be related to insufficient intervention dose (23), particularly in the Taveras et al. study, where only around 50% of the participants completed at least two of the six planned intervention contacts (22).
Motivational interviewing (MI), a client-centred communication style, has been used extensively to modify chronic disease risk factors in adults including obesity and is a recommended counselling style; however, its efficacy in paediatric obesity has only been examined in a few, generally small-scale studies (21,27–31). Finally, use of multidisciplinary teams, e.g. physician and dietician, has been recommended, but few studies have tested the impact of this approach (21,32), particularly using an additive design.
The current study, named BMI2 (Brief MI to reduce Body Mass Index), was designed to test the efficacy of two levels of MI intensity conducted by trained primary care physicians and trained dieticians. This paper reports the overall study design and describes the baseline cohort.
Design
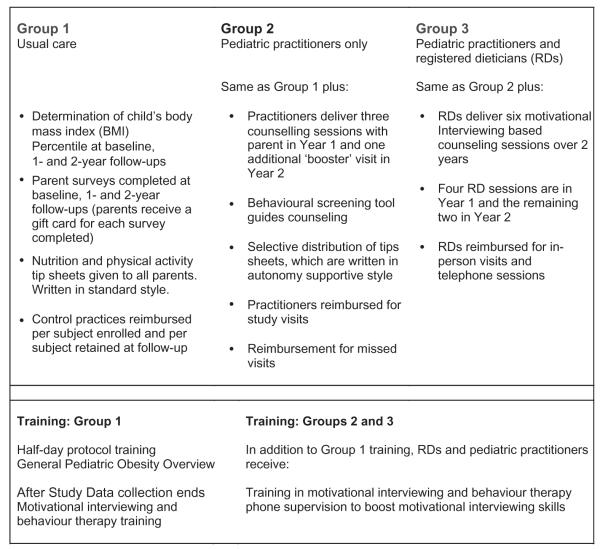
The study is a cluster-randomized intervention with clinical practices serving as the unit of randomization and analysis (Fig. 1). We are testing two increasingly intensive interventions compared with a minimal intensity/usual care (UC) group. Group 1 (UC) includes determination of BMI percentile at baseline, and at 1- and 2-year follow-ups and routine care by the primary care physician as well as standard educational materials provided to parents. UC paediatricians and their study staff received a 0.5 d study orientation session, which included a brief CME-type workshop addressing obesity treatment and an overview of current treatment guidelines (33,34). At the end of the study, UC practitioners will be offered the complete MI and behaviour change training and DVD materials. Group 2 includes the same assessment points as UC. In addition, Group 2 paediatric practitioners (PPs) received 2 d of in-person training in MI and behaviour therapy (BT) as well as an interactive DVD MI booster training system focusing on paediatric obesity. PPs in Group 2 are asked to schedule three proactive counselling sessions with a parent of the index child in Year 1 and one additional ‘booster’ visit in Year 2, although they were given some latitude in their appointment scheduling. To guide their counselling, they are provided with a food and activity screening tool adapted from the instrument used in our pilot study (21). In addition, Group 2 practices are provided with educational materials written in a style consistent with MI and the self determination theory, described later (35). Unlike in Group 1, where all of the educational materials are provided proactively to each parent, in Groups 2 and 3, materials are distributed on a more selective tailored basis depending on parent needs, requests and motivation. Group 3 (PP + registered dietician [RD]) includes the same intervention components as Group 2, but adds MI-based counselling from a trained, RD who is linked to that practice. RDs deliver six MI-based counselling sessions over 2 years. RDs were given the flexibility to schedule counselling session with families at their discretion, although again they were encouraged to provide more visits towards the beginning of the intervention. The RD sessions are delivered both in-person (required for visit 1) and optionally by telephone or in-person, subsequently. Similar to MDs, RDs received 2 d of in-person MI and BT training, and the interactive DVD MI booster training system. For some sections of the in-person MI and BT training, RDs met separately from MDs to discuss issues unique to their practice.
Figure 1.
Study overview Brief Motivational Interviewing to Reduce Body Mass Index2.
The study’s primary hypotheses are:
At 2-year follow-up, children in the moderate intensity intervention group (PP only) will show a three-point (absolute) decrease in BMI percentile relative to children in the minimal intensity/US group.
At 2-year follow-up, children in the high intensity intervention group (PP + RD) will show a three point (absolute) decrease in BMI percentile relative to children in the moderate intensity group (PP only).
Ethics approval was obtained from the University of Michigan and the American Academy of Paediatrics (AAP). Most Pediatric Research in Office Settings (PROS) practices (n = 37) operated under the umbrella of the AAP Review Board (IRB) whereas the remaining practices (n = 5), obtained local IRB approval. All sites had IRB approval before initiating subject recruitment.
Primary outcome, sample size and statistical approach
The primary outcome is the child’s BMI percentile at 2-year follow-up. Secondary outcomes include parent report of the child’s screen time, physical activity, intake of fruits and vegetables, sugar-sweetened beverages and family meals. Cost effectiveness analyses will also be conducted.
Sample size calculations assumed a three-point difference in BMI percentile between Groups 3 and 2 and Groups 2 and 1 at 2-year follow-up, with a standard deviation for BMI percentile between four and six; power of 0.80 and two tailed alpha of 0.05. We accounted for the cluster randomized design, using the method recommended by Murray (36,37), assuming a practice level intraclass correlation (ICC) between 0.01 and 0.05. Based on the assumptions above and projected 25% attrition at 2-year followup, with 10–12 practices per arm (30–36 total) and 15–20 children per practice at baseline, the study will be adequately powered for our primary outcome.
Outcome analysis
The primary outcome will be BMI percentile at 2-year follow-up. We will assume an intention-to-treat approach, with individuals analyzed in the condition they were assigned. Repeated measures analysis of variance (ANOVA), appropriate for nested data, will be the primary model. In general, analyses will include factors for group (three levels: US = 0, PP only = 1 and PP/RD = 2), the within-subjects repeated measures factor of time (three levels: baseline, follow-up 1 and follow-up 2) and the group × time interaction. Potential covariates include: child’s gender, age and ethnicity as well as parent’s age and baseline BMI. To control for cluster randomization effects, we will utilize mixed effects regression with individuals nested within their practice.
Process measures
At the end of the study, a subset of two parents from each intervention practice will be interviewed by telephone to gauge their overall reactions to the project, perceived impact on their child’s weight, diet, physical activity, impact on family behaviour, and reaction to their paediatrician (Group 2) and paediatrician and RD (Group 3) counsellors. Furthermore, all practitioners and RDs will be interviewed as well to address their overall reaction to the project protocol, impact of the training on their practice, reaction to the reimbursement (Groups 2 and 3), perceived reaction on the part of parents and children (Groups 2 and 3), recommendations for improvement of the intervention (Groups 2 and 3) and use of the DVD.
Baseline measurements
BMI percentile
As part of the protocol training for all three experimental groups, PPs and their office staff assistant who attended the training, were shown proper assessment of height and weight, and provided with print tools to convert heights/weights to BMI and BMI percentile. We ensured that all practices were accurately measuring height by sending a 36′ calibration rod. For practices that did not record accurate heights, a new stadiometer was also sent. We also sent practices a digital scale to use for the study. Parent BMI (an exploratory outcome and potential covariate) is calculated from self-reported heights and weights.
Parent questionnaire
Parents complete a questionnaire at baseline, and at 1- and 2-year follow-ups. The questionnaire assesses a range of behavioural and psychosocial variables, described later. The primary objective for the parent questionnaire is to provide both the PPs and RDs with starting points for their behavioural counselling. Additionally, the instrument is being used to provide data for secondary outcomes.
Fruit and vegetables
How many servings of fruits (fresh fruit, frozen fruit, canned fruit, but NOT including juice) does your child eat on a typical day? A serving is about 8 oz, or one medium piece of fruit, or one half-cup of raw fruit. How many servings of vegetables (fresh, frozen or canned, but NOT including potatoes) does your child eat on a typical day? A serving is about one half-cup of cooked vegetables, or one cup of raw vegetables.
Sweetened drinks
For each of the following drinks, how many glasses or 12 oz cans does your child drink on a typical day? There were four options; fruit drinks (such as Hi-C, Hawaiian Punch, lemonade, Koolaid Capri-Sun), sports drinks (such as Gatorade), regular soda and sweet tea.
Physical activity
On a typical weekday/weekend day, how many hours is your child involved in sports or active play?
Responses were none/less than 1 h per day, 1–2 h, 2–3 h and over 3 h. We recoded 1–2 h per day as 1.5 h and 2–3 as 2.5 h. Weekend and weekday hours were averaged to estimate total activity per day.
Screen time
How many hours of TV does your child watch on a typical weekday/weekend day including evenings? How many hours of games (computer, Xbox, Wii, DS, Playstation and internet games like Webkinz) does your child play on a typical weekday/weekend day including evenings? Responses were; None/less than 1 h per day, 1–2 h, 2–3 h, 3–4 h, 4–5 h, Over 5 h. We recoded 1–2 h per day as 1.5 h, 2–3 as 2.5 h, 3–4 as 3.5 h and 4–5 as 4.5 h. Weekend and weekday hours were averaged to estimate total screen time per day.
Other items included; about how many times per week does your family eat together at home? and does your child have a TV in his/her room?
Parent programme expectations
Parental outcome expectations for the programme were assessed with four items (i) My child will succeed in achieving a healthy weight; (ii) My family will be able to make changes in our eating; (iii) My family will be able to make changes in our physical activity; and (iv) My family will be able to make changes in our TV/video/computer use. Responses ranged from 0–10, with 0 not at all sure and 10 very sure.
Parent rating of child behaviour
For the primary target behaviours we asked parents to rate their child on a scale from A (great/healthy) to F (poor/unhealthy). The eight behaviours were; snack foods, sweetened beverages, eating out at restaurants, fruits, vegetables, TV/screen time, video games/compute games and physical activity/ exercise. These ratings are used by clinicians in Groups 2 and 3 to help set the counselling agenda. Herein, we report the percent responding A vs. all other grades combined.
Demographics
Parents were asked to report household income using eight contiguous categories. These categories were collapsed into ‘less than $40 000’ and ‘greater than or equal to $40 000’ to simplify presentation. Education was assessed with seven exclusive categories, which were collapsed into ‘less than college graduate’ and ‘college graduate or greater’. We asked about the child’s insurance coverage first by querying if the child had any insurance, and then by asking about specific types, e.g. private, Medicaid, SCHIP.
Recruiting practices
Paediatric Research in Office Settings: the PROS network
All study sites were recruited from the AAP PROS network. PROS is a practice-based research network established in 1986 by the AAP (38). Currently, PROS is the largest paediatric primary care research network in the nation, comprising 1741 practitioners from 737 paediatric practices in 50 states, Puerto Rico and Canada. Based on estimates of patients per practitioner derived from a previous PROS study, (39) PROS practitioners provide care for more than 3 million children in the US. The PROS network has an extensive record of conducting successful primary care research and addressing a wide range of topics (40–47). Practitioners include MDs as well as Nurse Practitioners, therefore we term this group.
Paediatric practitioners
Based on an unpublished comparison of current PROS membership data and a random national sample of AAP generalists gathered from 2008– 2010, PROS practitioners are somewhat older (median 51 years vs. 46 years), more likely to be to male (43% vs. 41%) and more likely to practice in an urban or rural area (58% vs. 42%).
We approached PROS sites that had previously participated in at least one prior research project. We excluded sites that offered a structured obesity treatment programme or those where the participating clinician had extensive experience with MI. During recruitment of study sites, each practice was asked to identify an office staff member who would serve as the local study coordinator. This person attended the protocol training.
Identifying and recruiting dieticians
RDs were selected from a registry of practising dieticians within the American Dietetic Association’s, Dietetics Practice-Based Research Network (DPBRN). None of the RDs were, at the time of their recruitment, working at the medical practices recruited for the study. We paired the RDs with a practice, although in a few instances the RD and local physician may have had some prior professional contact. Once identified as living and practising within a reasonable range of a paediatric practice randomized to Group 3, RDs were interviewed to assess their potential for implementing MI. To assess potential MI skills, RDs were presented with a simulated patient encounter. We rated how well they were able to listen without immediately offering dietary advice and to roll with resistance rather than counter argue with the simulated patient. Following this standardized interviewing procedure RDs chosen to be a part of the study were invited to participate in a 2-d Ml training workshop with their designated PP and clinic coordinator.
A total of 13 RDs were initially recruited and all attended their initial MI training. One RD dropped out before seeing any patients, and a 14th was added and trained to replace her.
Target population
The target population is children ages 2–8 years old with a BMI ≥ 85th and ≤97th percentile based on CDC cutpoints (48). We exclude youth above the 97th percentile of BMI because at higher levels of BMI, many clinicians would initiate metabolic screening and likely refer the child to a paediatric endocrinologist and/or cardiologist. Also, metabolic diagnostics and referral practices would likely differ substantially across primary care practices, which would introduce a potential study confound. Additionally, requiring such testing from all practices would entail significant cost to the study. Finally, some of the clinicians from the our pilot felt that even with the training and support provided by the study, they would not feel comfortable treating such extremely overweight youth and that such patients needed specialist care. All practices were asked to enrol at least 20 and up to 25 eligible children. Given the higher rates of overweight and obesity in minority children, we oversampled practices with at least 25% Black and/or Hispanic patients. Our goal was to accrue approximately 5% Black and 25% Hispanic patients.
Exclusion Criteria for children include Type 1 or Type II diabetes, non-English speaking parent, no working telephone, children in foster care or group homes, children with chronic medical disorders, chromosomal disorders, syndromes and non-ambulatory conditions (such as myelodysplasia, cerebral palsy), children taking medications known to affect growth and mood (e.g. select psychotropic such as selective serotonin reuptake inhibitors, stimulants, oral steroids or other growth hormones, enrolment in a weight loss programme or seen by weight loss specialist in past 12 months. Eligibility was initially determined by the study practices and then confirmed by the study team. Those enrolled by practices but subsequently found to be ineligible by the study team were allowed to continue in the study but their data will not be included in any analyses.
Intervention
Target behaviours and intervention strategies in Groups 2 and 3
Both PPs and RDs are trained to focus their counselling on discrete dietary and activity behaviours that have been shown to affect children’s weight in observational and/or intervention studies (49–53). Specifically, we target snack foods, sweetened beverages, eating in restaurants, fruits, vegetables, TV/screen time, video games/computer games and physical activity/exercise.
Target behaviours are identified with a brief screener created for this project. For each of the domains above parents indicate the frequency of the behaviour as well as Grade (discussed previously), and clinicians are provided with a scoring template that codes parent responses as red, yellow or green. Clinicians are asked to provide positive feedback for ‘green’ behaviours and then collaboratively with the parent, identify red or yellow behaviours that the parent would be willing to discuss and possibly modify. Dieticians were provided with baseline questionnaire responses to further assist them in their counselling.
MI and behavioural skills training
Primary care physicians and dieticians received a 2-d interactive training in MI by the first author. The workshop included five didactic elements:
Conceptual overview of MI: its spirit and essential strategies
Comparison of MI with other models of counselling and patient education
••
Conceptual application of MI to prevention and treatment of paediatric obesity, including nuances of working with parents of young children as well as working directly with older children
Integration of MI with anticipatory guidance and dietary therapy approaches.
Clinical skills were addressed through interactive exercises and video around six areas:
Constructing effective open-ended questions
Reflective listening
- Eliciting change talk
- Use of the 0–10 importance and confidence rulers.
- Building discrepancy through values clarification
Handling ambivalence and resistance
Providing information and advice MI-style
Closing the deal and follow-up.
As part of the training, practitioners were also taught basic behaviour modification skills related to weight control such as self-monitoring, goal setting and autonomy support. The overall framework for the behavioural model was based on principles of self-regulation and self-determination theory (54). We taught clinicians to help parents support their child’s autonomy by providing them with a range of healthy choices such as which type of cereal to eat and which type of fruit or vegetable to eat as well as involving them in food shopping and recipe selection. At the end of the 2-d training, all clinicians counselled standardized patients, typically played by study staff. These encounters were video-taped and rated with a standardized MI fidelity scale (available from the first author). While clinicians were at the training, they received detailed feedback from study staff about their counselling encounter. Practitioners were offered an additional supervision session by telephone. To help practitioners build their skills, they were provided with a DVD that summarized the basic principles and strategies of MI and demonstrated several MI encounters around paediatric obesity.
Educational materials
For the US group, clinicians distributed a set of generally pre-existing, educational materials that addressed healthy eating and exercise. All UC parents received the same set of materials. For Groups 2 and 3, we chose pre-existing materials or created new materials written in a style consistent with MI and self-determination theory. For example, content emphasized parent and child choice in making behaviour change and information was generally presented without fear messages. Groups 2 and 3 also were offered self-monitoring logs for the child and/or parent to complete. For Groups 2 and 3, clinicians offered only those materials that were either requested by the patient or that related to the target behaviour change that was chosen by the family.
Lack of reimbursement is at least a moderate barrier to prevention and treatment. Given that the study is fundamentally an efficacy trial, we felt it important to neutralize this barrier to allow a cleaner test of the counselling interventions without the confounding variable of physician adherence due to reimbursement. Therefore, PPs in both Groups 2 and 3 as well as RDs in Group 3 are provided with compensation. PPs receive $50 per session. PPs in Groups 2 and 3 will conduct four visits over 2 years with each family (three in Year 1 and one in Year 2). Therefore, total reimbursement will be $200 per completed case for PPs. RDs are compensated $50 per in-person visit and $35 for telephone sessions. To account for reports of high ‘no-show’ rates in paediatric practices, we allowed a $25 reimbursement for scheduled appointments that the patient failed to keep, to a total of $250 per practitioner (PP or RD). There are also incentives for practice participation. Groups 2 and 3 receive $500 upon initiating the study, and $1000 upon completion. Group 1 receives $25 per child enrolled and $50 per child retained at Year 2 along with a start-up incentive of $250 and completion incentive of $500.
Results
A total of 42 practices have been recruited and randomized. From these, a total of 662 children were recruited, of which 29 were excluded because upon verification by study staff their BMI fell outside the target range of 85th to 97th percentile. Results are reported here for the remaining 633 eligible enrolled children.
One practice dropped out because the primary clinician died and one practice recruited no patients. Of the remaining 40 practices, the mean number of eligible patients recruited was 15.8 (range; 2–25). Average time to recruit was 234 d (range; 30–481 d). Since recruitment began, one additional practice dropped out because the primary clinician moved to a different practice.
Demographics
Table 1 presents the demographics of the cohort. Mean BMI percentile was 92.0, with a range of 85–97. The groups were equivalent for our primary outcome. The ICC because of clinic level clustering for BMI percentile was 0.019. Mean age of the cohort was 5.1, with a range of 2–8 years. The three study groups were significantly different with regard to child age, with Groups 2 and 3 recruiting older children than Group 1. Parent BMI, calculated from their self-reported heights and weights, did not differ by group.
Table 1.
Baseline cohort demographics Brief Motivational Interviewing to Reduce Body Mass Index22
| % | Group 1 Usual care (n = 214) |
Group 2 PP MI (n = 200) |
Group 3 PP + RD MI (n = 219) |
Total (n = 633) |
|---|---|---|---|---|
| Mean child age (SD)* | 4.8 (1.7) | 5.1 (1.9) | 5.3 (1.8) | 5.1 (1.8) |
| Mean child BMI percentile (SD) | 91.6 (3.3) | 92.2 (3.3) | 92.0 (3.5) | 92.0 (3.4) |
| Parent BMI (SD) | 27.9 (7.2) | 29.4 (8.2) | 27.9 (7.1) | 28.4 (7.5) |
| Child gender | ||||
| Female | 52.3 | 57.5 | 61.0 | 57.0 |
| Parent completing questionnaire* | ||||
| Mother | 88.3 | 93.0 | 91.7 | 91.0 |
| Father | 11.3 | 4.0 | 7.3 | 7.6 |
| Other | 0.5 | 3.0 | 0.9 | 1.4 |
| Child race† | ||||
| White | 71.2 | 56.3 | 60.0 | 62.5 |
| Black | 3.4 | 11.7 | 6.9 | 7.2 |
| Hispanic | 14.9 | 30.5 | 23.5 | 22.8 |
| Asian | 10.6 | 1.5 | 9.7 | 7.4 |
| Household income | ||||
| <$ 40 000 | 28.2 | 36.5 | 29.5 | 30.9 |
| ≥$ 40 000 | 71.8 | 63.5 | 71.5 | 69.1 |
| Parent education* | ||||
| <College | 63.9 | 68.5 | 52.1 | 61.2 |
| College or higher | 36.1 | 31.5 | 47.9 | 38.8 |
| Child insurance coverage | ||||
| Any | 99.5 | 98.0 | 96.3 | 97.9 |
| Private | 73.2 | 63.3 | 68.1 | 68.3 |
| Medicaid† | 16.4 | 34.2 | 23.3 | 24.4 |
Study groups differ P < 0.05, based on ANOVA for continuous and chi-square for categorical variables.
Study groups differ P < 0.01, based on ANOVA for continuous and chi-square for categorical variables.
MI, motivational interviewing; PP, paediatric practitioners; RD, registered dietician; SD, standard deviation; anova, analysis of variance.
The cohort was 57% female and 91% of the responding parents were mothers. Groups 2 and 3 had a greater percentage of mothers as respondents than Group 1. Overall, almost 70% of parents reported household income greater than or equal to $40 000 per year and 39% reported at least a college education. Education differed by study group with Group 3 having the highest college completion rate. There were no group differences in the percent of parents reporting any insurance or private insurance by group, although Groups 2 and 3 had a higher percent reporting Medicaid coverage. With regard to ethnicity/race, the cohort was 63% White, 23% Hispanic, 7% Black and 7% Asian, and the three groups differed significantly with regard to ethnic/racial composition.
Behaviours and attitudes
As shown in Table 2, parents reported an average of 2.5 h of daily screen time (combined television and video games) for their children and 2 h of total daily physical activity. The groups significantly differed for screen time, with Group 2 having the highest mean as well as for physical activity, with Group 3 reporting the fewest hours per day. Approximately 1/3 of children have a television in their room, with Group 2 having the highest rate, 41%. Mean intake was 2.1 servings for fruit, 1.7 servings of vegetables and 1.0serving (cans) of sugared beverages, with significant group differences only for sugared drinks.
Table 2.
Baseline cohort psychosocial and behavioural variables Brief Motivational Interviewing to Reduce Body Mass Index22
| Group 1 Usual care (n = 214) |
Group 2 PP MI (n = 200) |
Group 3 PP + RD MI (n = 219) |
Total (n = 633) |
|
|---|---|---|---|---|
| Daily§ hours of screen time (SD)† | 2.3 (1.4) | 2.7 (1.5) | 2.3 (1.4) | 2.5 (1.4) |
| Television in bedroom (% yes)* | 32.9 | 40.7 | 27.9 | 33.6 |
| Daily hours of physical activity (SD)* | 2.1 (0.76) | 2.0 (0.74) | 1.9 (.79) | 2.0 (0.77) |
| Daily servings fruits (SD) | 2.1 (1.1) | 2.0 (.98) | 2.1 (1.2) | 2.1 (1.1) |
| Daily servings vegetables (SD) | 1.7 (1.0) | 1.6 (1.0) | 1.7 (1.1) | 1.7 (1.0) |
| Daily cans sugared beverages (SD)† | 0.77 (1.1) | 1.4 (1.7) | 0.99 (1.5) | 1.0 (1.5) |
| Confidence‡ child will achieve healthy weight | 8.2 (2.1) | 7.8 (2.3) | 8.0 (2.3) | 8.0 (2.2) |
| Confidence‡ family will change eating | 7.9 (2.3) | 8.0 (1.9) | 8.1 (2.0) | 8.1 (2.1) |
| Confidence‡ family will change activity | 8.0 (2.2) | 8.1 (2.1) | 8.3 (1.8) | 8.1 (2.0) |
| Confidence‡ family will change screen time | 7.8 (2.5) | 7.6 (2.5) | 7.8 (2.4) | 7.7 (2.5) |
| Perceived child behavioural grade (% reporting A) | ||||
| Snack foods | 17.5 | 13.2 | 12.6 | 14.4 |
| Sweetened beverages | 46.4 | 42.8 | 38.1 | 42.4 |
| Eating out | 28.7 | 26.3 | 23.6 | 26.2 |
| Fruits | 48.6 | 43.9 | 50.4 | 47.9 |
| Vegetables | 30.8 | 28.3 | 28.7 | 29.3 |
| TV/screen time | 23.1 | 15.7 | 17.9 | 18.9 |
| Video/compute games | 58.6 | 59.4 | 56.3 | 58.0 |
| Physical activity/exercise† | 41.1 | 21.3 | 22.3 | 28.4 |
Study groups differ P < 0.05, based on ANOVA for continuous and chi-square for categorical variables.
Study groups differ P < 0.01, based on ANOVA for continuous and chi-square for categorical variables.
0–10 with 0 not at all sure and 10 very sure.
Sum of television and video game time.
MI, motivational interviewing; PP, paediatric practitioners; RD, registered dietician; SD, standard deviation; anova, analysis of variance.
Parent self-reported confidence that their child will achieve a healthy weight was on average an 8 (out of 10). The mean was also around eight for the remaining three confidence variables, with no group differences in any of the four confidence variables.
Finally, the percentage of parents self-grading their child’s behaviour as an ‘A’ ranged from a low of 14.4% for snack foods to a high of 58% for video/ computer games. The only variable for which a group difference was observed was physical activity, with Group 1 showing the highest percent of ‘A’ grades.
Discussion
This study will be one of the largest clinical trials of a behavioural intervention to treat paediatric obesity in a primary care setting. Although the outcomes will not be known for 2 years or so, to date, several aspects of the study may nonetheless inform other similar efforts. First, the approach of using, in effect, volunteer clinicians to recruit participants was generally successful. Most practices accrued their sample target of 20 families.
There have been some unanticipated challenges, however, in this regard. One practitioner died in an accident before any participants were recruited from his site. In another practice, the team could not identify any eligible patients. In addition, two dieticians from Group 3 dropped out, one for personal reasons and one for career reasons. Delayed or insufficient communication from primary care practitioner offices to their associated RDs (e.g. faxing patient information) has, in a few practices impeded timely delivery of the RD counselling. Another issue is that a few sites have had personnel changes (e.g. practitioner changed practices) that significantly impeded recruitment and/or led to their discontinuing with the study. Underlying economic conditions also impacted recruitment, as in many practices, there was a larger number of patients losing insurance coverage than in previous years.
One rate-limiting step that may impede dissemination of MI in clinical practice is the willingness of practitioners to dedicate sufficient time to attend training programmes. And, the cost of ‘in-servicing’ practitioners may be prohibitive. All of our Group 2 and 3 practitioners who were recruited attended their 2-d MI workshop. The project paid for their travel and ground expenses but we did not pay them any stipend for attending their training. We did not pay them, in part because we expected they would find such skills inherently beneficial to their general practice. Although PROS practitioners may not represent all clinicians nationally, their willingness to attend and fully participate in the MI workshop without pay provides some evidence that disseminating MI into clinical practice may be more feasible than previously thought; that practitioners, including RDs, may be intrinsically motivated to learn these skills and therefore willing to invest their time and energy to acquire them. To bring such a model to scale, cost-effective means to train practitioners that accommodate their clinical and administrative responsibilities will be needed, such as online self guided and webcast training, telephonic supervision and self-help training materials. The self-help DVD developed for this project is available from the first author. System changes, such as reimbursement for obesity counselling even absent comorbidities or using practicelevel BMI percentiles or delivery of obesity counselling as quality indicators, might also facilitate adoption of such a model. Finally, reimbursement policies and practice management strategies that encourage integration of dieticians and other behaviour change specialists into paediatric primary care settings may be needed.
Although BMI percentile, our primary outcome, did not differ between study groups at baseline, there were several variables, including both demographics and secondary outcomes, for which between-group baseline differences were observed including race, child age, parent gender, parent education, Medicaid status, screen time and sugared beverage intake. Baseline non-equivalence is a known problem in cluster randomized designs (36,55). Typically, had this been an individual randomization design, with 600 plus subjects, baseline equivalence would have been far more likely, if not a certainty. A practical implication of these findings is that these variables will be included, at least in initial models, as covariates. Another finding related to the practice homogeneity is the observed ICC. Accounting for the ICC is important to achieve adequate power in cluster randomized designs. Power calculations assumed an ICC between 0.01 and 0.05. The observed ICC was 0.019, which indicates that power should be adequate to account for the effect of cluster randomization.
Overall parents appeared to express strong confidence in their ability to execute change for their index child as well as their families, with average scores around 8 on a 10-point scale. In adults, although unrealistic weight loss expectations have not been associated with weight loss outcomes (56,57), they have been associated with higher programme attrition (58). Whether parental expectations will impact either outcomes or attrition in this study will be examined as the study unfolds.
The socioeconomic characteristics of the sample were somewhat skewed towards middle and upper socioeconomic status families, with over 70% earning above $40 000, 39% having college education and 68% having private insurance. The model being used here assumes continuity of care, which fits well for those with private insurance. However, given continuity of care is less common among those who are uninsured or publically insured, adaptations to the model would be needed for these populations. This may include engaging obesity or behaviour change specialists outside of primary care or limiting the number of sessions, when continuity of care is not possible. With regard to the content of the intervention for low-resource populations, greater emphasis on environmental factors, such as safety and lack of physical activity facilities and attention to issues such as food insecurity, affordability of food and transportation to care may be needed (59,60).
With regard to paying for services, the model used herein reimburses PPs and RDs for their counselling, albeit somewhat modestly. Current reimbursement policies vary considerably by state, by payers, and by insurers; however, there has been in recent years an increase in the number of insurers, e.g. Blue Cross Blue Shield as well as Medicaid in North Carolina (61), that reimburse for obesity counselling, even absent co-morbidities. The Affordable Care Act, if fully enacted would also promote better coverage for paediatric obesity counselling (62). In the meantime, guidelines to help practitioners obtain greater reimbursement for obesity counselling by using existing billing codes more effectively have also been developed (63).
To help clinicians determine the counselling agenda with parents, we developed a parent self-report grading scheme. One initial concern was that there would be little variability in parental scores, and in particular, overly positive scoring on the part of parents. The distribution of ‘A’ grades, which ranged from 14.4% for snack foods to 58% for video/ computer games, suggests this rating system does yield adequate variability.
Although the primary use of the parent questionnaire is to guide clinicians’ counselling, many of the variables in the parent questionnaire will also be used for secondary analyses. The validity of such brief, somewhat more qualitative dietary and physical activity measures may only be modest (64–68). Therefore, we are conducting a separate validity study to examine the association of our measures with multiple 24-h recalls and accelerometer data. Many of the measures and procedures used in this study, which can be obtained from the first author, can be used by other practitioners and researchers.
Footnotes
Conflict of Interest Statement No conflict of interest was declared.
References
- 1.Ogden CL, Carroll MD, Curtin LR, Lamb MM, Flegal KM. Prevalence of high body mass index in US children and adolescents, 2007–2008. JAMA. 2010;303:242–249. doi: 10.1001/jama.2009.2012. [DOI] [PubMed] [Google Scholar]
- 2.Lee WW. An overview of pediatric obesity. Pediatr Diabetes. 2007;8(Suppl. 9):76–87. doi: 10.1111/j.1399-5448.2007.00337.x. [DOI] [PubMed] [Google Scholar]
- 3.Finkelstein E, Fiebelkorn I, Wang G. National medical spending attributable to overweight and obesity: how much, and who’s paying? Health Aff. 2003;W3:219–226. doi: 10.1377/hlthaff.w3.219. [DOI] [PubMed] [Google Scholar]
- 4.Wolf A, Colditz GA. Current estimates of the economic cost of obesity in the United States. Obes Res. 1998;6:97–106. doi: 10.1002/j.1550-8528.1998.tb00322.x. [DOI] [PubMed] [Google Scholar]
- 5.Must A, Spadano J, Coakley EH, Field AE, Colditz G, Dietz WH. The disease burden associated with overweight and obesity. JAMA. 1999;282:1523–1529. doi: 10.1001/jama.282.16.1523. [DOI] [PubMed] [Google Scholar]
- 6.Dietz W. Health consequences of obesity in youth: childhood predictors of adult disease. Pediatrics. 1998;101:518–525. [PubMed] [Google Scholar]
- 7.Rhodes ET, Ebbeling CB, Meyers AF, et al. Pediatric obesity management: variation by specialty and awareness of guidelines. Clin Pediatr (Phila) 2007;46:491–504. doi: 10.1177/0009922806298704. [DOI] [PubMed] [Google Scholar]
- 8.Miller JL, Silverstein JH. Management approaches for pediatric obesity. Nat Clin Pract Endocrinol Metab. 2007;3:810–818. doi: 10.1038/ncpendmet0669. [DOI] [PubMed] [Google Scholar]
- 9.Story MT, Neumark-Stzainer DR, Sherwood NE, et al. Management of child and adolescent obesity: attitudes, barriers, skills, and training needs among health care professionals. Pediatrics. 2002;110:210–214. [PubMed] [Google Scholar]
- 10.Kolagotla L, Adams W. Ambulatory management of childhood obesity. Obes Res. 2004;12:275–283. doi: 10.1038/oby.2004.35. [DOI] [PubMed] [Google Scholar]
- 11.Perrin EM, Flower KB, Garrett J, Ammerman AS. Preventing and treating obesity: pediatricians’ self-efficacy, barriers, resources, and advocacy. Ambul Pediatr. 2005;5:150–156. doi: 10.1367/A04-104R.1. [DOI] [PubMed] [Google Scholar]
- 12.Jelalian E, Boergers J, Alday CS, Frank R. Survey of physician attitudes and practices related to pediatric obesity. Clin Pediatr (Phila) 2003;42:235–245. doi: 10.1177/000992280304200307. [DOI] [PubMed] [Google Scholar]
- 13.Epstein LH, Paluch RA, Roemmich JN, Beecher MD. Family-based obesity treatment, then and now: twenty-five years of pediatric obesity treatment. Health Psychol. 2007;26:381–391. doi: 10.1037/0278-6133.26.4.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Magarey AM, Perry RA, Baur LA, et al. A parent-led family-focused treatment program for overweight children aged 5 to 9 years: the PEACH RCT. Pediatrics. 2011;127:214–222. doi: 10.1542/peds.2009-1432. [DOI] [PubMed] [Google Scholar]
- 15.Nowicka P, Flodmark CE. Family in pediatric obesity management: a literature review. Int J Pediatr Obes. 2008;3(Suppl. 1):44–50. doi: 10.1080/17477160801896994. [DOI] [PubMed] [Google Scholar]
- 16.Kalarchian MA, Levine MD, Arslanian SA, et al. Family-based treatment of severe pediatric obesity: randomized, controlled trial. Pediatrics. 2009;124:1060–1068. doi: 10.1542/peds.2008-3727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Resnicow K, Yaroch A, Davis A, et al. GO GIRLS!: results from a pilot nutrition and physical activity program for low-income overweight African American adolescent females. Health Educ Behav. 2000;27:633–648. doi: 10.1177/109019810002700507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Resnicow K, Taylor R, Baskin M. Results of Go Girls: a nutrition and physical activity intervention for overweight African American adolescent females conducted through Black churches. Obes Res. 2005;13:1739–1748. doi: 10.1038/oby.2005.212. [DOI] [PubMed] [Google Scholar]
- 19.Epstein LH, Paluch RA, Beecher MD, Roemmich JN. Increasing healthy eating vs. reducing high energy-dense foods to treat pediatric obesity. Obesity (Silver Spring) 2008;16:318–326. doi: 10.1038/oby.2007.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taveras EM, Hohman KH, Price SN, et al. Correlates of participation in a pediatric primary care-based obesity prevention intervention. Obesity (Silver Spring) 2011;19:449–452. doi: 10.1038/oby.2010.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schwartz RP, Hamre R, Dietz WH, et al. Office-based motivational interviewing to prevent childhood obesity: a feasibility study. Arch Pediatr Adolesc Med. 2007;161:495–501. doi: 10.1001/archpedi.161.5.495. [DOI] [PubMed] [Google Scholar]
- 22.Taveras EM, Gortmaker SL, Hohman KH, et al. Randomized controlled trial to improve primary care to prevent and manage childhood obesity: the high five for kids study. Arch Pediatr Adolesc Med. 2011;165:714–22. doi: 10.1001/archpediatrics.2011.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McCallum Z, Wake M, Gerner B, et al. Outcome data from the LEAP (Live, Eat and Play) trial: a randomized controlled trial of a primary care intervention for childhood overweight/mild obesity. Int J Obes (Lond) 2007;31:630–636. doi: 10.1038/sj.ijo.0803509. [DOI] [PubMed] [Google Scholar]
- 24.Woolford SJ, Sallinen BJ, Clark SJ, Freed GL. Results from a clinical multidisciplinary weight management program. Clin Pediatr (Phila) 2011;50:187–191. doi: 10.1177/0009922810384845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saelens BE, Sallis JF, Wilfley DE, Patrick K, Cella JA, Buchta R. Behavioral weight control for overweight adolescents initiated in primary care. Obes Res. 2002;10:22–32. doi: 10.1038/oby.2002.4. [DOI] [PubMed] [Google Scholar]
- 26.Whitlock EP, O’Connor EA, Williams SB, Beil TL, Lutz KW. Effectiveness of weight management interventions in children: a targeted systematic review for the USPSTF. Pediatrics. 2010;125:e396–e418. doi: 10.1542/peds.2009-1955. [DOI] [PubMed] [Google Scholar]
- 27.Resnicow K, Davis R, Rollnick S. Motivational interviewing for pediatric obesity: conceptual issues and evidence review. J Am Diet Assoc. 2006;106:2024–2033. doi: 10.1016/j.jada.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 28.Flattum C, Friend S, Neumark-Sztainer D, Story M. Motivational interviewing as a component of a school-based obesity prevention program for adolescent girls. J Am Diet Assoc. 2009;109:91–94. doi: 10.1016/j.jada.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brennan L, Walkley J, Fraser SF, Greenway K, Wilks R. Motivational interviewing and cognitive behaviour therapy in the treatment of adolescent overweight and obesity: study design and methodology. Contemp Clin Trials. 2008;29:359–375. doi: 10.1016/j.cct.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 30.Carels RA, Darby L, Cacciapaglia HM, et al. Using motivational interviewing as a supplement to obesity treatment: a stepped-care approach. Health Psychol. 2007;26:369–374. doi: 10.1037/0278-6133.26.3.369. [DOI] [PubMed] [Google Scholar]
- 31.Irby M, Kaplan S, Garner-Edwards D, Kolbash S, Skelton JA. Motivational interviewing in a family-based pediatric obesity program: a case study. Fam Syst Health. 2010;28:236–246. doi: 10.1037/a0020101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ross MM, Kolbash S, Cohen GM, Skelton JA. Multi-disciplinary treatment of pediatric obesity: nutrition evaluation and management. Nutr Clin Pract. 2010;25:327–334. doi: 10.1177/0884533610373771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barlow SE. Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: summary report. Pediatrics. 2007;120(Suppl. 4):S164–S192. doi: 10.1542/peds.2007-2329C. [DOI] [PubMed] [Google Scholar]
- 34.Spear BA, Barlow SE, Ervin C, et al. Recommendations for treatment of child and adolescent overweight and obesity. Pediatrics. 2007;120(Suppl. 4):S254–S288. doi: 10.1542/peds.2007-2329F. [DOI] [PubMed] [Google Scholar]
- 35.Resnicow K, Davis RE, Zhang G, et al. Tailoring a fruit and vegetable intervention on novel motivational constructs: results of a randomized study. Ann Behav Med. 2008;35:159–169. doi: 10.1007/s12160-008-9028-9. [DOI] [PubMed] [Google Scholar]
- 36.Murray D. Design and Analysis of Group Randomized Trials. Oxford University Press; New York: 1998. [Google Scholar]
- 37.Murray D, Wolfinger R. Analysis issues in the evaluation of community trials: progress toward solutions in SAS/ STAT MIXED. J Community Psychol. 1994;22:140–145. [Google Scholar]
- 38.Wasserman R, Slora E, Bocian A, et al. Pediatric Research in Office Settings (PROS): a national practice-based research network to improve children’s health care. Pediatrics. 1998;102:1350–1357. doi: 10.1542/peds.102.6.1350. [DOI] [PubMed] [Google Scholar]
- 39.Bocian A, Wasserman R, Slora E, Kessel D, Miller R. Size and age-sex distribution of pediatric practice: a study from Pediatric Research in Office Settings [see comments] Arch Pediatr Adolesc Med. 1999;153:9–14. doi: 10.1001/archpedi.153.1.9. [DOI] [PubMed] [Google Scholar]
- 40.Wasserman RC, Croft CA, Brotherton SE. Preschool vision screening in pediatric practice: a study from the Pediatric Research in Office Settings (PROS) Network. American academy of pediatrics [published erratum appears in Pediatrics 1992 Dec;90(6):1001] Pediatrics. 1992;89:834–838. [PubMed] [Google Scholar]
- 41.Taylor J, Darden P, Brooks D, et al. Impact of the change to inactivated poliovirus vaccine on the immunization status of young children in the United States: a study from Pediatric Research in Office Settings and the National Medical Association. Pediatrics. 2001;107:e90. doi: 10.1542/peds.107.6.e90. [DOI] [PubMed] [Google Scholar]
- 42.Taylor J, Darden P, Slora E, Hasemeier C, Asmussen L, Wasserman R. The influence of provider behavior, parental characteristics, and a public policy initiative on the immunization status of children followed by private pediatricians: a study from Pediatric Research in Office Settings. Pediatrics. 1997;99:209–215. [PubMed] [Google Scholar]
- 43.Forrest C, Glade G, Starfield B, Baker A, Kang M, Reid R. Gatekeeping and referral of children and adolescents to specialty care. Pediatrics. 1999;104:28–34. doi: 10.1542/peds.104.1.28. [DOI] [PubMed] [Google Scholar]
- 44.Pantell R, Newman T, Bernzweig J, et al. Management and outcomes of care of fever in early infancy. JAMA. 2004;291:1203–1212. doi: 10.1001/jama.291.10.1203. [DOI] [PubMed] [Google Scholar]
- 45.Gardner W, Kelleher K, Wasserman R, et al. Primary care treatment of pediatric psychosocial problems: a report from PROS and ASPN. Pediatrics. 2000;106:e44. doi: 10.1542/peds.106.4.e44. [DOI] [PubMed] [Google Scholar]
- 46.PROS studies violence, child abuse prevention. AAP News. 2003 Apr;Sect.:143. •• ••. [Google Scholar]
- 47.Herman-Giddens M, Slora E, Wasserman R, et al. Secondary sexual characteristics and menses in young girls seen in office practice: a study from the Pediatric Research in Office Settings network. Pediatrics. 1997;99:505–512. doi: 10.1542/peds.99.4.505. [DOI] [PubMed] [Google Scholar]
- 48. [accessed •• 2011];BMI calculator. •• ••. [WWW document]. URL http://apps.nccd.cdc.gov/dnpabmi/
- 49.Davis MM, Gance-Cleveland B, Hassink S, Johnson R, Paradis G, Resnicow K. Recommendations for prevention of childhood obesity. Pediatrics. 2007;120:S229–S253. doi: 10.1542/peds.2007-2329E. [DOI] [PubMed] [Google Scholar]
- 50.American Dietetic Association’s Evidence Analysis Library [accessed June 2011];Factors associated with childhood overweight. [WWW document]. URL http://www.adaevidencelibrary.com/topic.cfm?cat=4156.
- 51.Flynn MA, McNeil DA, Maloff B, et al. Reducing obesity and related chronic disease risk in children and youth: a synthesis of evidence with ‘best practice’ recommendations [see comment] Obes Rev. 2006;7(Suppl. 1):7–66. doi: 10.1111/j.1467-789X.2006.00242.x. [DOI] [PubMed] [Google Scholar]
- 52.Ludwig DS, Peterson KE, Gortmaker SL. Relation between consumption of sugar-sweetened drinks and childhood obesity: a prospective, observational analysis. Lancet. 2001;357:505–508. doi: 10.1016/S0140-6736(00)04041-1. [DOI] [PubMed] [Google Scholar]
- 53.Bray GA, Nielsen SJ, Popkin BM. Consumption of high-fructose corn syrup in beverages may play a role in the epidemic of obesity. Am J Clin Nutr. 2004;79:537–543. doi: 10.1093/ajcn/79.4.537. [DOI] [PubMed] [Google Scholar]
- 54.Ryan RM, Deci EL. Self-determination theory and the facilitation of intrinsic motivation, social development, and well-being. Am Psychol. 2000;55:68–78. doi: 10.1037//0003-066x.55.1.68. [DOI] [PubMed] [Google Scholar]
- 55.Varnell SP, Murray DM, Janega JB, Blitstein JL. Design and analysis of group-randomized trials: a review of recent practices. Am J Public Health. 2004;94:393–399. doi: 10.2105/ajph.94.3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fabricatore AN, Wadden TA, Womble LG, et al. The role of patients’ expectations and goals in the behavioral and pharmacological treatment of obesity. Int J Obes. 2007;31:1739–1745. doi: 10.1038/sj.ijo.0803649. [DOI] [PubMed] [Google Scholar]
- 57.Linde JA, Jeffery RW, Finch EA, Ng DM, Rothman AJ. Are unrealistic weight loss goals associated with outcomes for overweight women? Obes Res. 2004;12:569–576. doi: 10.1038/oby.2004.65. [DOI] [PubMed] [Google Scholar]
- 58.Dalle Grave R, Calugi S, Molinari E, et al. Weight loss expectations in obese patients and treatment attrition: an observational multicenter study. Obes Res. 2005;13:1961–1969. doi: 10.1038/oby.2005.241. [DOI] [PubMed] [Google Scholar]
- 59.Kumanyika S, Grier S. Targeting interventions for ethnic minority and low-income populations. Future Child. 2006;16:187–207. doi: 10.1353/foc.2006.0005. [DOI] [PubMed] [Google Scholar]
- 60.Estabrooks PA, Glasgow RE. Translating effective clinic-based physical activity interventions into practice. Am J Prev Med. 2006;31:S45–S56. doi: 10.1016/j.amepre.2006.06.019. [DOI] [PubMed] [Google Scholar]
- 61.Heubeck E. Reimbursement offers hope for more obesity counseling. DOC News. 2007;4:8. [Google Scholar]
- 62. [accessed July 2011];Preventive services covered under the Affordable Care Act. •• ••. [WWW document]. URL http://www.healthcare.gov/law/about/provisions/services/lists.html.
- 63.American Academy of Pediatrics . Coding for Pediatric Preventive Care 2011. American Academy of Pediatrics; Elk Grove Village, IL: 2011. [Google Scholar]
- 64.Welk GJ, Corbin CB. The validity of the Tritrac-R3D Activity Monitor for the assessment of physical activity in children. Res Q Exerc Sport. 1995;66:202–209. doi: 10.1080/02701367.1995.10608834. [DOI] [PubMed] [Google Scholar]
- 65.McPherson RS, Hoelscher DM, Alexander M, Scanlon KS, Serdula MK. Dietary assessment methods among school-aged children: validity and reliability. Prev Med. 2000;31:S11–S33. [Google Scholar]
- 66.Field AE, Peterson KE, Gortmaker SL, et al. Reproducibility and validity of a food frequency questionnaire among fourth to seventh grade inner-city school children: implications of age and day-to-day variation in dietary intake. Public Health Nutr. 1999;2:293–300. doi: 10.1017/s1368980099000397. [DOI] [PubMed] [Google Scholar]
- 67.Huybrechts I, Vereecken C, De Bacquer D, et al. Reproducibility and validity of a diet quality index for children assessed using a FFQ. Br J Nutr. 2010;104:135–144. doi: 10.1017/S0007114510000231. [DOI] [PubMed] [Google Scholar]
- 68.Purslow LR, van Jaarsveld CH, Semmler C, Wardle J. Validity and prognostic value of parental ratings of children’s activity. Prev Med. 2009;49:28–31. doi: 10.1016/j.ypmed.2009.04.010. [DOI] [PubMed] [Google Scholar]