Table 2. Substrate scope of the [2 + 2] reaction a .
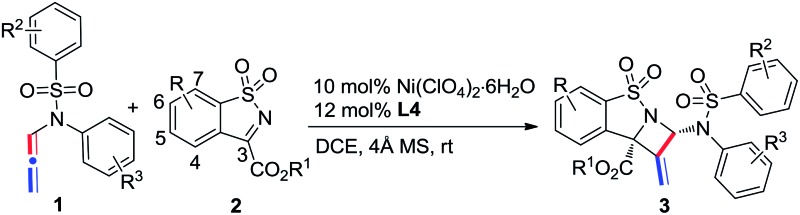
| ||||
| Entry | 1 (R2/R3) | 2 (R/R1) | Yield b (%) | ee c (%) |
| 1 | 1a (4-Me/H) | 2a (H/Et) | 82 (3aa) | 99 |
| 2 | 1a (4-Me/H) | 2b (H/iPr) | 75 (3ab) | 90 |
| 3 | 1a (4-Me/H) | 2c (H/Me) | 76 (3ac) | 99 |
| 4 | 1a (4-Me/H) | 2d (H/ n Bu) | 80 (3ad) | 98 |
| 5 | 1a (4-Me/H) | 2e (5-OMe/Et) | 77 (3ae) | 99 |
| 6 | 1a (4-Me/H) | 2f (5-Me/Et) | 73 (3af) | 97 |
| 7 | 1a (4-Me/H) | 2g (5- t Bu/Et) | 70 (3ag) | 98 |
| 8 | 1a (4-Me/H) | 2h (5-Cl/Et) | 64 (3ah) | 99 |
| 9 | 1a (4-Me/H) | 2i (5-F/Et) | 70 (3ai) | 95 |
| 10 | 1a (4-Me/H) | 2j (5-CF3/Et) | 52 (3aj) | 99 |
| 11 | 1a (4-Me/H) | 2k (5-OCF3/Et) | 55 (3ak) | 98 |
| 12 | 1a (4-Me/H) | 2l (7-Cl/Et) | 64 (3al) | 94 |
| 13 | 1a (4-Me/H) | 2m (7-OCF3/Et) | 76 (3am) | 93 |
| 14 | 1a (4-Me/H) | 2n (6,7-(CH)4/Et) | 70 (3an) | 94 |
| 15 | 1b (4-Me/4-Me) | 2a (H/Et) | 75 (3ba) | 99 |
| 16 | 1c (4-Me/4-Br) | 2a (H/Et) | 67 (3ca) | 97 |
| 17 | 1d (4-Me/3-OMe) | 2a (H/Et) | 78 (3da) | 99 |
| 18 | 1f (H/H) | 2a (H/Et) | 70 (3fa) | 99 |
| 19 | 1g (4- t Bu/H) | 2a (H/Et) | 80 (3ga) | 99 |
| 20 | 1h (4-OMe/H) | 2a (H/Et) | 70 (3ha) | 94 |
| 21 | 1i (4-CF3/H) | 2a (H/Et) | 66 (3ia) | 99 |
| 22 | 1j (3,4-(CH)4/H) | 2a (H/Et) | 80 (3ja) | 94 |
| 23 | 1k (4-Cl/H) | 2a (H/Et) | 72 (3ka) | 98 |
| 24 | 1k (4-Cl/H) | 2e (5-OMe/Et) | 75 (3ke) | 99 |
| 25 | 1k (4-Cl/H) | 2h (5-Cl/Et) | 90 (3kh) | 98 |
| 26 | 1b (4-Me/4-Me) | 2e (5-OMe/Et) | 85 (3be) | 98 |
a Reaction conditions are identical to those in entry 4 in Table 1.
b Isolated yield of the only isomer.
c Determined by chiral HPLC.
