Abstract
Cryopreservation of few spermatozoa is still a major challenge for male fertility preservation. This study reports use a new micro-straw (LSL straw) for freezing few spermatozoa for intracytoplasmic sperm injection (ICSI). Semen samples from 22 fertile donors were collected, and each semen sample was diluted and mixed with cryoprotectant in a ratio of 1:1, and then frozen using three different straws such as LSL straw (50–100 μl), traditional 0.25 ml and 0.5 ml straws. For freezing, all straws were fumigated with liquid nitrogen, with temperature directly reducing to −130–−140°C. Sperm concentration, progressive motility, morphology, acrosome integrity, and DNA fragmentation index were evaluated before and after freezing. After freezing-thawing, LSL straw group had significantly higher percentage of sperm motility than traditional 0.25 ml and 0.5 ml straw groups (38.5% vs 27.4% and 25.6%, P < 0.003). Sperm motility and acrosomal integrity after freezing-thawing were significantly lower than that of before freezing. However, there was no significant difference in morphology, acrosome, and DNA integrity between the three types of straws (P > 0.05). As LSL straws were thinner and hold very small volume, the freezing rate of LSL straw was obviously faster than 0.25 ml straw and 0.5 ml straws. In conclusion, LSL micro-straws may be useful to store few motile spermatozoa with good recovery of motility for patients undergoing ICSI treatment.
Keywords: cryopreservation, freezing rate, LSL straw, micro-straw, motility
INTRODUCTION
Intracytoplasmic sperm injection (ICSI) is currently a most effective treatment procedure for severe male factor infertility such as severe oligozoospermia or azoospermia if any sperm can be found in ejaculate or testicular biopsy specimens or epididymal sperm aspiration.1,2,3 At present, for clinical routine ICSI patients, the pregnancy rate per ICSI cycle was about 40%–45%.4,5 However, in some couples with severe male factor infertility, ICSI treatment cycle may be canceled due to no motile sperm found from semen or testicular biopsy specimens.6 Some patients need 2nd ICSI cycles with repeated sperm retrieval due to failed previous cycles. Repeated operations may not only bring psychological pressure and economic burden to male patients but also lead to the testicular damage, the epididymal fibrosis, irreversible testicular atrophy, spermatogenic function degradation, and even the loss of endocrine function which needs exogenous testosterone replacement therapy.7,8 Thus, a reliable and safe procedure for few spermatozoa cryopreservation was urgently required for oligozoospermic or azoospermic patients. However, the common techniques and protocols used for semen cryopreservation still have certain limitations, and there is still no effective method for clinical cryopreservation of few human spermatozoa.
Up to now, cryopreservation of few spermatozoa (2-3 items LPF−1) was studied by Cohen et al.9 and Desai et al.10 who used the zona pellucida as freezing carrier to store few sperm. Desai et al.11,12 adopted the cryoloop to cryopreserve testicular and epididymal sperm. Subsequently, Huang Weihua et al.13 reported cryopreservation of few spermatozoa using vitrification method without adding cryoprotectants. These studies yet had their pros and cons – existence of foreign proteins in zona pellucida may cause biological contamination, use of cryoloop costs too much (several cryoloops are needed for testicular and epididymal sperm), and open freezing straws without adding cryoprotectants may lead to liquid nitrogen pollution. This study aims to investigate a new enclosed mirco-straw (LSL straw, which is made of an outer metal shell and an inner tube) for cryopreservation of rare human spermatozoa, improving the security and quality of freeze-thaw sperm, thus contributing to undergoing ICSI treatment cycle with cryopreserved sperm for oligozoospermic or azoospermic patients.
MATERIALS AND METHODS
Sperm sample
Semen samples of 22 healthy donors (ages ranged from 22 to 30 years, mean, 24.6 ± 4.2 years) were randomly collected from Shanghai Human Sperm Bank, Ren Ji Hospital, School of Medicine, Shanghai Jiao Tong University. Semen was obtained by masturbation after an abstinence of 2–7 days. Then, a routine semen analysis was performed according to the World Health Organization (WHO) Laboratory Manual for the Examination and Processing of Human Semen (5th Edition), and parameters of these semen samples were within the following range: volume of semen >2 ml, sperm concentration >60 × 106 ml−1, and progressive motility rate (PR) >60%. This study has been approved by the Reproductive Ethics Committee of Ren Ji Hospital, and all donors have signed the written informed consent.
Three types of straws: LSL micro-straw, 0.25 ml and 0.5 ml straws
Micro-straws (LSL straws, Figure 1a, including an outer metal shell and an inner tube) were supplied by Cryo BioSystem, Paris, France. The inner tube (diameter, 0.6–0.8 mm, thickness 0.07 mm, and volume 100 μl) with a fine end (diameter, 0.2–0.28 mm) is made of transparent polymerized resin. Importantly, the outer metal shell contributes to accelerating low-temperature conduction and protecting the inner tube during freezing.
Figure 1.
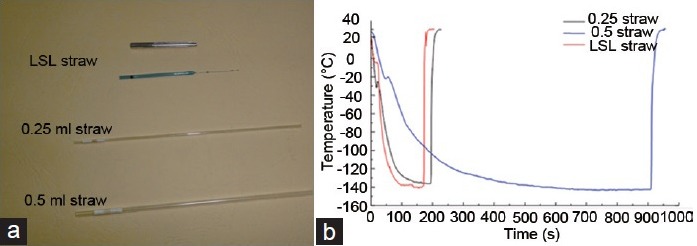
Difference between the cooling rates during liquid nitrogen fumigation of three types of straws. (a) Images of the structure of 0.25 ml straw, 0.5 ml straw, and LSL straw. Micro-straws (LSL straws) are made of an outer metal shell and an inner tube. (b) The cooling rate of three different types of straws. The cooling rate of LSL straw was significantly faster than that of 0.25 mL and 0.5 mL straws, and the cooling rates of 0.25 mL straws showed obviously faster than that of 0.5 mL straws, but with the similar trends of changes.
The 0.5 ml and 0.25 ml straws (Figure 1a), also supplied by Cryo BioSystem, Paris, France, are transparent tubes and widely used for sperm cryopreservation in sperm banks or reproductive centers.
Sperm preparation before freezing
Fresh semen samples were incubated at 37°C in a water bath for liquefaction. After liquefied, sperm concentration and motility were examined with Makler counting chamber (Sefi Medical Instruments, Haifa, Israel) by inverted phase-contrast microscope (Olympus BX43, Tokyo, Japan). Then, the semen was diluted to the concentration of 10 × 106 ml−1 with 5% human tubal fluid (HTF). For freezing, cryoprotectant solution was dropped to the diluted semen in ratio of 1:1. Modified GYC (glycerin - yolk - sodium citrate) was used as the cryoprotectant in this study, which is consisted of 2.0 g trehalase, 1.5 g fructose, 3.4 g sodium citrate, 1 g glycine, 15 ml glycerol, and 20 ml egg yolk in 100 ml distilled water. Moreover, the pH is 7.2.
The semen from each donor will be frozen with three types of straws, including LSL micro-straw, 0.25 ml and 0.5 ml straws.
Freezing and thawing
For LSL straws
As shown in Figure 2, first, several drops of 50 μl mixed semen and cryoprotectant (About 2.5 × 105 spermatozoa per drop) were placed into a culture dish (Figure 2a). We pushed up the outer metal shell of LSL straw and exposed the inner tube (Figure 2b) to aspirate mixed sample with a sterile syringe (Figure 2c), and then pushed down the outer metal shell to protect the pine end of the inner tube (Figure 2d). Finally, LSL straw (About 2.5 × 105 spermatozoa per straw) was placed into a freezing stent (fumigated with liquid nitrogen) before stored in liquid nitrogen at −196°C.
Figure 2.
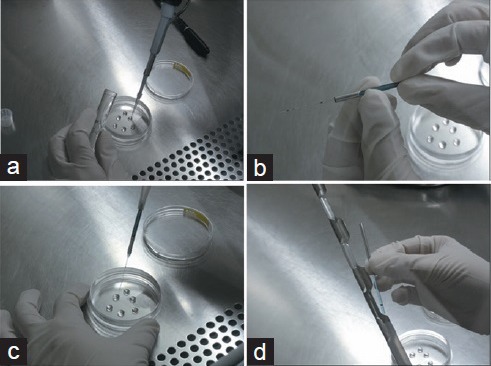
Steps of sperm freezing with the LSL straw. (a) Prepare micro-drop of mixed semen and cryoprotectant in a culture dish. (b) Move up the mental cover of LSL straw. (c) Suction of mixed sample into the LSL straw using 1 ml syringe attached rubber tube. (d) Placed LSL straw to a freezing stent for fumigating with liquid nitrogen.
For thawing of sperm, the LSL straws were exposed at room temperature for 10 s. The solution of the LSL straws was lightly blown into prewarmed recovery solution at 37°C in a 5% CO2 incubator for 10 min. Then, the concentration and motility of cryopreserved sperm were immediately examined with Makler counting chamber by an inverted microscope.
For 0.25 ml and 0.5 ml straws
The mixture of semen and cryoprotectant was drawn into 0.25 ml and 0.5 ml straws, and then the straws were sealed and directly fumigated in liquid nitrogen at −130–140°C for 3 h before transferred into liquid nitrogen for preservation.
The straws were thawed in a water bath at 37°C for 5 min. Then, we cut the sealed ends of the straws and lightly blew out the solution into prewarmed resuscitation drops. The following steps were the same with that of the LSL straw.
Detection of the cooling (temperature-descending) rate during freezing
In this study, agilent GL800 data acquisition instrument (provided by Institute of Refrigeration and Cryogenics, School of Mechanical Engineering, Shanghai Jiao Tong University) was adopted as the temperature apparatus, with T-type copper-constantan thermocouple as thermometric component. Both ends of the thermocouple were welded to form the temperature measuring points. Copper wire was the thermocouple's positive terminal with acquisition instrument's cathode, and constantan wire was the negative terminal with instrument's anode. To improve the measuring precision and accuracy, ice-point compensation method was conducted to monitor temperature changes during freezing and thawing.
To measure the cooling (temperature-descending) rate of the mixture in the straws during freezing and thawing, T-type thermocouple's temperature measurement point was placed into to-be-tested straws, which was fixed with cryogenic adhesive to prevent the probe drop-off. The to-be-tested straws were placed directly into fumigation liquid nitrogen analyzer and tested the cooling rate (temperature/s). When the temperature dropped to −150°C, the straws were thawed in a water bath at 37°C. Each straw was measured at least 3 times.
Determination of sperm morphology, acrosome integrity, and DNA fragmentation index
Sperm morphology was assessed by Diff-Quik staining (Biomart, Shenzhen, China) in the fresh and postthawing samples. Acrosome integrity was also assessed by a triple-fluorescence test (fluorescein isothiocyanate-conjugated Pisum sativum agglutinin staining, FITC-PSA staining). The sperm was incubated with FITC-PSA solution at 37°C for 30 min, and then observed with fluorescence microscope (Olympus, Tokyo, Japan). The DNA fragmentation index of sperm was performed using the Halosperm kit (sperm chromatin diffusion method, SCD method, Halotech DNA, SL, Madrid, Spain) according to the manufacturer's protocol.
Statistical analysis
The resuscitation rate was calculated by comparing forward progressive motility before and after freezing-thawing. The data of forward progressive motility rate (PR), sperm morphology, sperm acrosome integrity, and DNA fragmentation index were analyzed by the paired t-test (one-way variance analysis and Fisher LSD test) among these three groups.
RESULTS
Due to thin-wall and small-volume of LSL straw, heat conduction during freezing-thawing reached the fastest rate (Figure 1b). After freezing and thawing, sperm motility of the LSL straw showed obviously higher forward progressive motility and resuscitation rate (P < 0.003, Figure 3) than that of the 0.25 ml and 0.5 ml straws. In addition, LSL straw group also showed high normal morphology and normal acrosome and DNA integrity rate compared with the 0.25 ml and 0.5 ml straws. However, there was no difference in normal sperm morphology, normal acrosome integrity, and DNA fragmentation index between 0.25 ml and 0.50 ml straws (P > 0.05, Figures 3 and 4).
Figure 3.
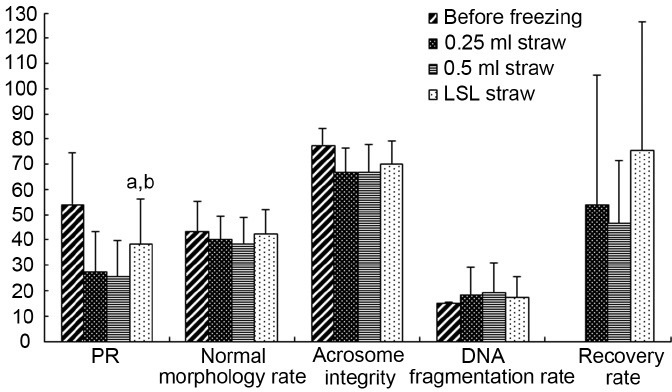
Semen parameters of samples before and after the freezing-thawing process in 0.25 ml straw, 0.5 ml straw, and LSL straw. aP < 0.05, comparison between LSL and 0.5 ml straws; bP < 0.05, comparison between LSL and 0.25ml straws.
Figure 4.
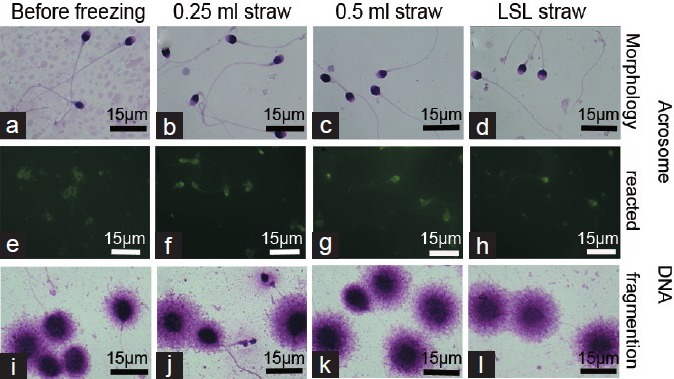
Assessment of sperm morphology, acrosome integrity, and DNA fragmentation index before and after freezing-thawing. (a–d) Morphology of sperm before and after freezing-thawing, no significant difference was observed in acrosome integrity (e–h) and DNA fragmentation index (i–l) of sperm between the 0.25 ml and 0.5 ml and LSL straws before and after freezing-thawing. Scale bars = 8 μm.
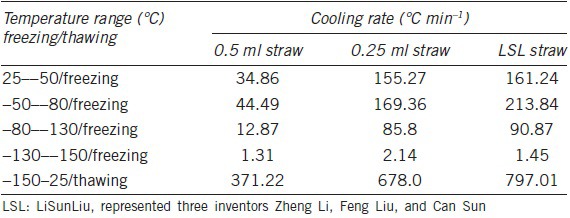
The cooling rate during sperm cryopreservation showed meaningful difference between the LSL, 0.25 ml and 0.5 ml straws (Table 1 and Figure 1b). During the freezing process, the cooling rate of LSL straw was obviously faster than 0.25 ml and 0.5 ml straws, showing 262.80, 153.45, and 42.99°C min−1 from −50°C to −80°C and 94.32°C min−1, 57.12°C min−1 and 12.71°C min−1 from −80°C to −130°C, respectively, thus rapidly getting through the freezing point, during which the intracellular ice and extracellular ice formed. The trends of changes in cooling rates were very similar between 0.25 ml and 0.5 ml straws whereas cooling rate of 0.25 ml straw is 2.7–4.5-folds of that of 0.5 ml straws.
Table 1.
Comparison of cooling rate during freezing (liquid nitrogen fumigation) and thawing between LSL straw, 0.25 ml and 0.5 ml straws
DISCUSSION
Recently, minimally invasive sperm retrieval techniques have achieved rapid progress with the applications of microsurgical technique, such as percutaneous epididymal sperm aspiration (PESA), testicular sperm extraction (TESE), and microsurgical epididymal sperm aspiration (MESA).14,15,16,17 These sperm retrieval techniques made it possible to successfully obtain few spermatozoa in azoospermia patients for further ICSI treatment. Sperm cryopreservation before oocyte collection plays a key role for further ICSI treatment and avoids repeating sperm extraction from epididymal or testicular. However, cryopreservation of few spermatozoa obtained from operation is still challenging. Therefore, it is necessary to develop an efficient procedure for cryopreserving few spermatozoa obtained from epididymis and testis by surgical sperm retrieval. In this study, sperm from the healthy donors was used as samples of few spermatozoa by diluting to the concentration of 10 × 106 ml−1 with 5% HTF, due to the rare and precious spermatozoa from azoospermia and oligoasthenospermia patients. Moreover, 5% HTF is widely used for human spermatozoa culturing and washing in clinical.
In this study, LSL straw improved sperm motility after freezing-thawing by reducing ice damage with rapid heat conduction during cryopreservation.18 The routine cryopreservation method with 0.25 ml and 0.5 ml straws is less optimal for cryopreservation of the few spermatozoa from severe oligozoospermic or azoospermic patients. The intracellular and extracellular ice formations during freezing are the major causes of cryoinjury for sperm motility and viability of IVF, vitrification method for cryopreservation of oocyte and embryo has gained great advance and is preferred to routine freeze-thaw method, showing almost complete avoidance of ice formation with the extremely rapid rate of cooling. Nijs and Ombelet19 and Liebermann et al.20 suggested that the cooling rate in the process of freezing was determined by cryoprotectant and carrier. The LSL straw adopted in this study consisting of an outer metal shell and an inner tube, showed rapid cooling rate during freezing compared with the 0.25 ml and 0.5 ml straws, thus reducing the ice formation in the process of temperature descending during fumigating with liquid nitrogen. Moreover, motility of postthaw sperm in the LSL straw group showed an obvious increase (PR 41%, 30% and 29%, P < 0.003), compared with 0.25 ml and 0.5 ml straws. The results of LSL straw group also showed high normal morphology rate, acrosome, and DNA integrity rates. Therefore, the micro-straw or LSL straw is preferred to 0.25 ml and 0.5 ml straws for clinical cryopreservation of a small group of spermatozoa, with the rapider cooling rate. The cooling rate of straws during freezing by fumigated with liquid nitrogen is dominated by the strength of heat flux from sample to liquid nitrogen and the cold transmission from liquid nitrogen to sample, according to the law of conservation of energy, which may explain the same trends of cooling rate of 0.25 ml and 0.5 ml straws during fumigation with liquid nitrogen gas.
CONCLUSION
Freezing-thawing sperm motility of the LSL straw was significantly higher than that of 0.25 ml and 0.5 ml straw. The LSL straw holds smaller volume than 0.25 and 0.50 ml straws; therefore, it is much easy to find few spermatozoa postfreezing and thaw. The micro-straw for freezing of testicular and epididymis sperm appears to be superior to conventional 0.25 ml and 0.5 ml straws.
AUTHOR'S CONTRIBUTIONS
FL, SSZ, and ZL performed conception or design of the work. YZ, CS, YFL, and WBS made substantial contributions to sample collection, freezing and thawing. SSW and YHH carried out Detection of the cooling (temperature-descending) rate for the work. SSZ, FL, and JJZ analyzed and interpreted the data, and drafted this work, and all authors revised it critically for important intellectual content and finally approved the version to be published.
COMPETING INTERESTS
All authors declared no conflicting financial interests in this study.
ACKNOWLEDGMENTS
The authors would like to thank Professor De-Yi Liu for his advice and review of this manuscript. The work was supported by Shanghai Municipal Hospital Appropriate Technology Project (Grant No. SHDC12014236), Shanghai Commission of Family Planning (Grant No. 2013JG08) the National Research Program of China (Grant No. 2015AA020404), and the Foundation of Renji Hospital, School of Medicine, Shanghai Jiao Tong University (Grant No. RJZZ14-017) and the National Natural Science Foundations of China (Grant No. 81501243).
REFERENCES
- 1.Ohlander S, Hotaling J, Kirshenbaum E, Niederberger C, Eisenberg ML. Impact of fresh versus cryopreserved testicular sperm upon intracytoplasmic sperm injection pregnancy outcomes in men with azoospermia due to spermatogenic dysfunction: a meta-analysis. Fertil Steril. 2014;101:344–9. doi: 10.1016/j.fertnstert.2013.10.012. [DOI] [PubMed] [Google Scholar]
- 2.Tsai YR, Lan KC, Tsai CC, Lin PY, Kung FT, et al. Pregnancy outcome and neonatal data of children born after intracytoplasmic sperm injection with a different duration of cryopreservation of spermatozoa obtained through testicular sperm extraction. Taiwan J Obstet Gynecol. 2013;52:329–34. doi: 10.1016/j.tjog.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 3.Di Santo M, Tarozzi N, Nadalini M, Borini A. Human sperm cryopreservation: update on techniques, effect on DNA integrity, and implications for ART. Adv Urol 2012. 2012 doi: 10.1155/2012/854837. 854837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Desai N, Goldberg J, Austin C, Sabanegh E, Falcone T. Cryopreservation of individually selected sperm: methodology and case report of a clinical pregnancy. J Assist Reprod Genet. 2012;29:375–9. doi: 10.1007/s10815-012-9733-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mantikou E, Youssef MA, van Wely M, van der Veen F, Al-Inany HG, et al. Embryo culture media and IVF/ICSI success rates: a systematic review. Hum Reprod Update. 2013;19:210–20. doi: 10.1093/humupd/dms061. [DOI] [PubMed] [Google Scholar]
- 6.Bernie AM, Ramasamy R, Stember DS, Stahl PJ. Microsurgical epididymal sperm aspiration: indications, techniques and outcomes. Asian J Androl. 2013;15:40–3. doi: 10.1038/aja.2012.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schlegel PN, Su LM. Physiological consequences of testicular sperm extraction. Hum Reprod. 1997;12:1688–92. doi: 10.1093/humrep/12.8.1688. [DOI] [PubMed] [Google Scholar]
- 8.Andersson AM, Jørgensen N, Frydelund-Larsen L, Rajpert-De Meyts E, Skakkebaek NE. Impaired leydig cell function in infertile men: a study of 357 idiopathic infertile men and 318 proven fertile controls. J Clin Endocrinol Metab. 2004;89:3161–7. doi: 10.1210/jc.2003-031786. [DOI] [PubMed] [Google Scholar]
- 9.Cohen J, Garrisi GJ, Congedo-Ferrara TA, Kieck KA, Schimmel TW, et al. Cryopreservation of single human spermatozoa. Hum Reprod. 1997;12:994–1001. doi: 10.1093/humrep/12.5.994. [DOI] [PubMed] [Google Scholar]
- 10.Desai N, Glavan D, Goldfarb J. A convenient technique for cryopreservation of micro-quantities of sperm. Fertil Steril. 1998;70:228. [Google Scholar]
- 11.Desai NN, Blackmon H, Goldfarb J. Single sperm cryopreservation on cryoloops: an alternative to hamster zona for freezing individual spermatozoa. Reprod Biomed Online. 2004;9:47–53. doi: 10.1016/s1472-6483(10)62109-8. [DOI] [PubMed] [Google Scholar]
- 12.Isachenko V1, Isachenko E, Rahimi G, Krivokharchenko A, Alabart JL, et al. Cryopreservation of human ovarian tissue by direct plunging into liquid nitrogen: negative effect of disaccharides in vitrification solution. Cryo Letters. 2002;23:333–44. [PubMed] [Google Scholar]
- 13.Zou Y, Yin T, Chen S, Yang J, Huang W. On-chip cryopreservation: a novel method for ultra-rapid cryoprotectant-free cryopreservation of small amounts of human spermatozoa. PLos One. 2013;8:e61593. doi: 10.1371/journal.pone.0061593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lewin A, Reubinoff B, Porat-Katz A, Weiss D, Eisenberg V, et al. Testicular fine needle aspiration: the alternative method for sperm retrieval in non-obstructive azoospermia. Hum Reprod. 1999;14:1785–90. doi: 10.1093/humrep/14.7.1785. [DOI] [PubMed] [Google Scholar]
- 15.Esteves SC, Miyaoka R, Agarwal A. Sperm retrieval techniques for assisted reproduction. Int Braz J Urol. 2011;37:570–83. doi: 10.1590/s1677-55382011000500002. [DOI] [PubMed] [Google Scholar]
- 16.Colpi GM, Piediferro G, Nerva F, Giacchetta D, Colpi EM, et al. Sperm retrieval for intra-cytoplasmic sperm injection in non-obstructive azoospermia. Ital J Urol Nephrol. 2005;57:99–107. [PubMed] [Google Scholar]
- 17.Timpano M, Fontana D, Rolle L, Preto M, Falcone M, et al. Sperm collection for medically assisted procreation in azoospermic patients. Urologia. 2014;81:27–31. doi: 10.5301/RU.2014.11984. [DOI] [PubMed] [Google Scholar]
- 18.Peng QP, Cao SF, Lyu QF, Xue SG, Jin W, et al. A novel method for cryopreservation of individual human spermatozoa. In vitro cellular and developmental biology. Animal. 2011;47:565–72. doi: 10.1007/s11626-011-9428-1. [DOI] [PubMed] [Google Scholar]
- 19.Nijs M, Ombelet W. Cryopreservation of human sperm. Hum Fertil (Camb) 2001;4:158–63. doi: 10.1080/1464727012000199232. [DOI] [PubMed] [Google Scholar]
- 20.Liebermann J, Nawroth F, Isachenko V, Isachenko E, Rahimi G, et al. Potential importance of vitrification in reproductive medicine. Biol Reprod. 2002;67:1671–80. doi: 10.1095/biolreprod.102.006833. [DOI] [PubMed] [Google Scholar]


