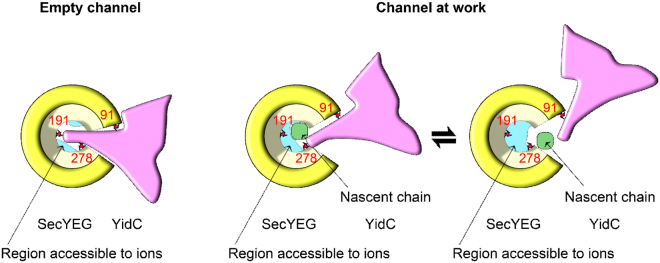
Figure 7.
Model of the YidC-SecYEG interaction as visualized by crosslinks and electrophysiological experiments. Left Panel: The empty SecYEG-YidC complex allows YidC to crosslink to the three indicated positions (red) at SecYEG’s pore ring (yellow) or the lateral gate. Ribosome binding is required to elicit the formation of a transmembrane channel (cross-section in blue) that allows ion permeation at low membrane potentials. Right Panel: Upon insertion of a nascent chain (green), YidC is expelled to the outer rim of the SecYEG pore, thereby increasing the cross-section (ion conductivity) of the SecYEG channel. Crosslinks to the channel interior (I191) is sterically constrained in the presence of a nascent chain while crosslinks to the peripheral residues 91 and 278 are still observable. In order to release the nascent chain, the lateral gate must open, i.e. conceivably the contact between SecYEG and YidC is further weakened. This is in line with crosslink data showing conformational changes at the SecY(I91)-YidC interface20. In summary, YidC facilitates the partitioning of a nascent membrane protein into the lipid environment by reducing the hydrophobicity of the lateral gate.